Host responses to bacterial infection are protective, yet without timely resolution they can lead to uncontrolled infection, with systemic engagement and multiple organ failure. In PNAS, Sekheri et al. (1) report the property of specific lipid mediators to counteract the delay tactics of bacterial DNA released from proliferating or dying bacteria as well as mitochondrial DNA released from injured cells during the host response against infection. The authors previously demonstrated that unmethylated CpG motifs, which are highly prominent in bacterial DNA, are recognized by the pathogen recognition receptor TLR9 and prolong neutrophil survival by suppressing cytochrome c release and limiting caspase-3 activation, the main executioner of apoptosis (2). Within their current work, they further explore the underlying mechanisms and discover that the specialized proresolving lipid mediators 15-epi-lipoxin A4 (15-epi-LXA4) and 17-epi-resolvin D1 (17-epi-RvD1) can counterregulate these signals and afford protection for the host (1). The researchers revealed an unexpected action for CpG DNA, which phosphorylates the Src kinase Hck associated with primary granule mobilization in the neutrophil. This leads to the release of two enzymes known to play a role in the inflammatory response, namely neutrophil elastase (NE) and proteinase 3 (PR3), although a direct link was not proven. In the presented experimental settings, NE and PR3 cleave the complement receptor C5aR from the plasma membrane, impacting negatively on bacterial uptake by neutrophils (a process termed phagocytosis): Inefficient phagocytosis alters the fate of these neutrophils and delays the resolution of inflammation. At the same time, impaired resolution favors persistence of infection with more marked damage to the host (3).
The processes of neutrophil apoptosis and the subsequent clearance of these cells by macrophages, termed efferocytosis, are regarded as hallmarks in the resolution of inflammation (4). If neutrophil apoptosis does not take place in a timely manner, then this can propagate infection and worsen the inflammatory response. Contrary to original belief, an acute inflammatory response does not simply dissipate due to dilution/degradation of proinflammatory mediators. Extensive work over the last couple of decades has defined that the resolving phase of inflammation is not a passive process, but actively “turns off inflammation” via the biosynthesis of endogenous antiinflammatory and proresolving mediators (5). These include proteins (e.g., annexin A1 and galectin-1), gaseous mediators (e.g., hydrogen sulfide and carbon monoxide), as well as specialized proresolving lipid mediators (SPMs) (e.g., lipoxins, resolvins, protectins, and maresins) (6).
SPMs are generated in response to infection by Gram-negative or Gram-positive bacteria as a natural mechanism to prevent self-damage, ensure resolution, and enhance survival (7). In line with this knowledge, Sekheri et al. (1) found that this was also true in a self-resolving murine model of pulmonary inflammation induced by intratracheal instillation of live Escherichia coli, which peaked at 6 h and resolved within 48 h postinfection. The authors were able to measure increased levels of 15-epi-LXA4 and RvD1 in bronchial lavage fluids at 6 h post E. coli infection, signifying that active resolution mechanisms are switched on.
The course of the host response to E. coli infection is delayed when CpG DNA was administered to mice via intraperitoneal injection: Such an intervention delayed bacterial clearance, suppressed polymorphonuclear leukocyte (PMN) apoptosis, impacted on efferocytosis, and ultimately reduced generation of proresolving lipid mediators. The end point of these effects was prolongation of lung disease with marked inflammation and infection at the 48-h time point, when mice infected solely with E. coli would be in the recovery phase.
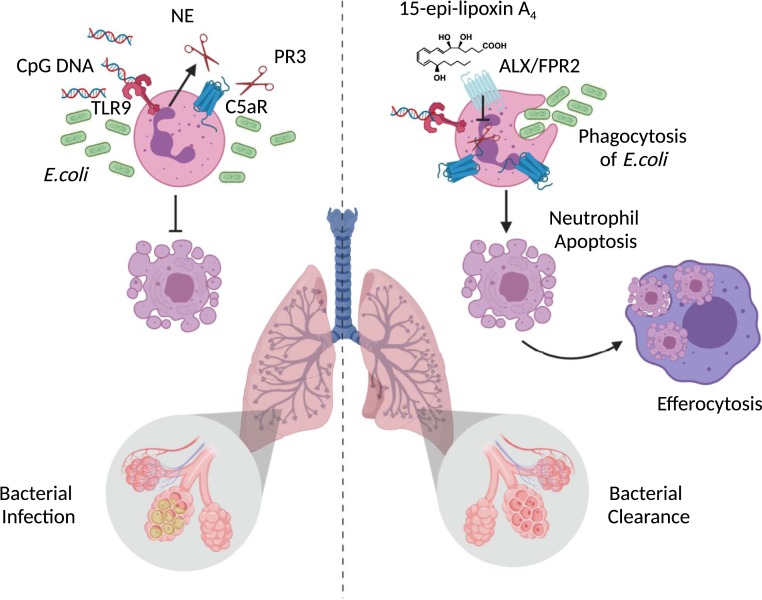
Importantly, the reduced generation of SPMs was not a bypass nonimpactful event, since exogenous treatment of mice at the peak of inflammation with either 15-epi-LXA4 or 17-epi-RvD1 at 6 h post E. coli plus CpG DNA accelerated clearance of bacteria. The authors then demonstrated that the two SPMs signaled via a specific G-protein–coupled receptor (ALX/FPR2) to repress the release of the enzymes NE and PR3 that normally spike in response to inflammation, and in turn prevent C5aR shedding (Fig. 1). Confirmation of ALX/FPR2 engagement by 15-epi-LXA4 and 17-epi-RvD1 on human PMN was determined using the pharmacological peptide antagonists Boc-2 and WRW4. The host-protective effects were elicited by submicromolar concentrations of SPMs. Moreover, the molecular actions provoked by SPM application restored neutrophil phagocytosis of bacteria and permitted neutrophil apoptosis to take place, thus expediting resolution and ensuring a proper host control of the infection generated by E. coli plus CpG DNA. In other words, application of SPMs overrides the negative effect produced by TLR9 activation, suggesting that agonism at ALX/FPR2 is a viable approach in settings of infection and danger signaling.
Fig. 1.
Specialized proresolving lipid mediators (SPMs) 15-epi-LXA4 and 17-epi-RvD1 restore impaired bacterial clearance and accelerate resolution of lung inflammation. Bacterial DNA (CpG DNA) are sensed via the pathogen recognition receptor TLR9 on neutrophils, which causes release of neutrophil elastase (NE) and proteinase 3 (PR3) and the subsequent cleavage of complement receptor (C5aR). This in turn impairs phagocytosis of E. coli and delays neutrophil apoptosis, leading to prolonged lung inflammation and bacterial infection (Left-Hand Side). By signaling through a specific G-protein–coupled receptor (GPCR), termed ALX/FPR2, 15-epi-LXA4 and 17-epi-RvD1 counterregulate signals from CpG DNA, allowing phagocytosis of E. coli and permitting neutrophils to undergo apoptosis, thereby promoting bacterial clearance and the resolution of pulmonary inflammation (Right-Hand Side).
Sekheri et al. (1) unveil new biology in the context of SPM regulation of the host response during infection. So far, infections have been combatted with species-specific therapies, antibiotics, or antivirals, depending on the nature of the infective agents. This study is aligned with the discoveries made by the J. G. Filep laboratory over the years, with the definition of the inhibition of apoptosis by proinflammatory agents, like serum amyloid protein, and the counteracting effect of proresolving mediators, including lipoxins and annexin A1 (8). Herein, the group provides evidence linking the protective properties of 15-epi-LXA4 or 17-epi-RvD1, which restore the cellular and tissue defects imposed by CpG DNA through TLR9 activation. By blocking the release of serine proteases, the two SPMs preserve the host function and prevent the shedding of C5aR, enabling a concerted and effective host response. The process of neutrophil apoptosis and subsequent efferocytosis by macrophages are essential in resolution (Fig. 1, Right-Hand Side).
ALX/FPR2 agonists can be developed for this potential clinical application too (9). Together with recent studies where specific SPMs have been shown to be an effective cotherapy in conjunction with antibiotics (7) or antiviral drugs (10), the data from Sekheri et al. (1) open therapeutic avenues for modes of controlling and regulating our response to infective agents. The study in PNAS by Sekheri et al. (1) provides further substance to the notion that proresolving-based therapy could be harnessed to fortify the host response in infective status (11, 12): This is of great potential and broad application to several infectious diseases that affect the world.
Acknowledgments
This work was supported by Versus Arthritis Senior Research Fellowship 22235, Barts Charity Project Grant MGU0443, Wellcome Trust Programme Grant 086867/Z/08/Z, and Versus Arthritis Project 21274. We used the BioRender software for generating the figure.
Footnotes
The authors declare no competing interest.
See companion article, “15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation,” 10.1073/pnas.1920193117.
References
- 1.Sekheri M., Kebir D. E., Edner N., Filep J. G., 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc. Natl. Acad. Sci. U.S.A. 117, 7971–7980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.József L., Khreiss T., Filep J. G., CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. FASEB J. 18, 1776–1778 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Maderna P., Godson C., Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim. Biophys. Acta 1639, 141–151 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Savill J., Apoptosis in resolution of inflammation. J. Leukoc. Biol. 61, 375–380 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Serhan C. N., Levy B. D., Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perretti M., Cooper D., Dalli J., Norling L. V., Immune resolution mechanisms in inflammatory arthritis. Nat. Rev. Rheumatol. 13, 87–99 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Chiang N., et al. , Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Kebir D., et al. , Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: A novel mechanism for resolution of inflammation. J. Immunol. 179, 616–622 (2007). [DOI] [PubMed] [Google Scholar]
- 9.El Kebir D., József L., Filep J. G., Opposing regulation of neutrophil apoptosis through the formyl peptide receptor-like 1/lipoxin A4 receptor: Implications for resolution of inflammation. J. Leukoc. Biol. 84, 600–606 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Morita M., et al. , The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 153, 112–125 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Perretti M., Leroy X., Bland E. J., Montero-Melendez T., Resolution pharmacology: Opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lee C. R., Zeldin D. C., Resolvin infectious inflammation by targeting the host response. N. Engl. J. Med. 373, 2183–2185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]