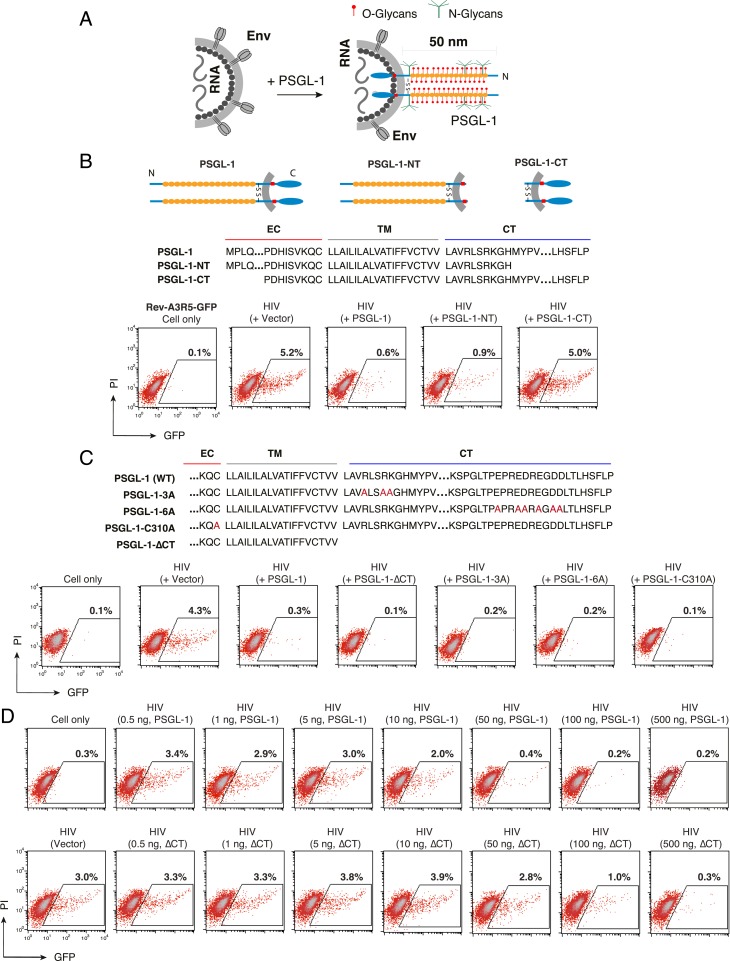
Fig. 4.
The extracellular N-terminal domain of PSGL-1 is required to block HIV-1 infectivity. (A) Hypothetical model by which virion incorporation of PSGL-1 restricts virion infectivity. Based on this model, incorporation of the heavily glycosylated and elongated PSGL-1 on viral particles may interfere with virion binding to target cells. (B) PSGL-1 domains involved in blocking HIV-1 infectivity. HEK293T cells were cotransfected with HIV(NL4-3) DNA (1 μg) plus vectors expressing PSGL-1 or PSGL-1 truncation mutants PSGL-1-NT or PSGL-1-CT (500 ng). Virions were harvested at 48 h posttransfection and normalized for p24, and viral infectivity was quantified by infecting Rev-A3R5-GFP indicator cells. HIV-1 replication was quantified by GFP expression. (C) PSGL-1 intracellular domain mutants PSGL-1-∆CT, PSGL-1-3A, and PSGL-1-6A and the dimerization mutant PSGL-1-C310A were similarly tested. EC, extracellular domain; TM, transmembrane domain; CT, cytoplasmic tail. (D) The PSGL-1-∆CT mutant displays reduced antiviral activity relative to WT PSGL-1. HEK293T cells were cotransfected with HIV(NL4-3) DNA (1 μg) plus various amounts of PSGL-1 or PSGL-1-∆CT (0.5 to 500 ng). Virions were harvested at 48 h and normalized for p24, and their infectivity was measured in Rev-A3R5-GFP indicator cells.