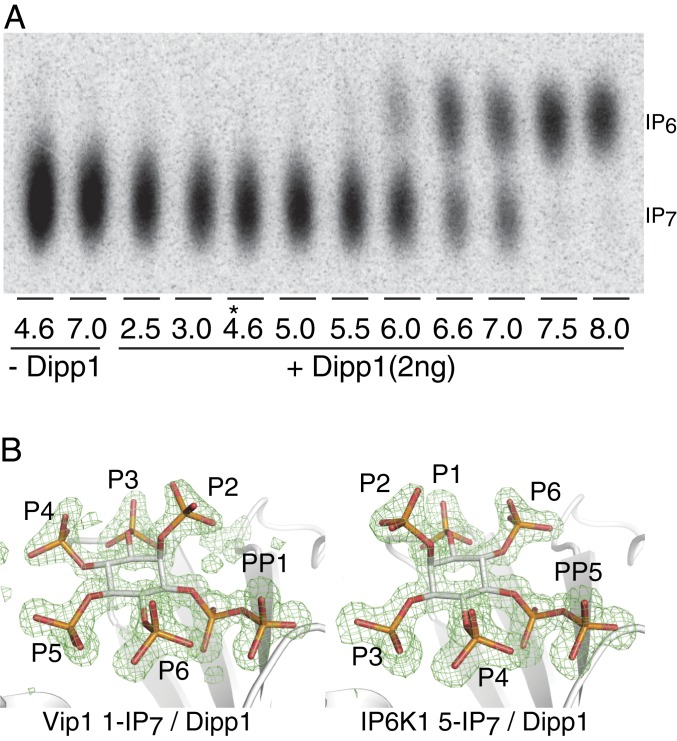
Fig. 1.
Identification of the product of scVIP1 as 1-IP7 through structure analysis of a cocrystal with hDipp1. (A) pH dependence of the Dipp1 1-IP7 pyrophosphatase reaction. The 7.5 µM Vip1 IP7 was incubated with 2 ng of hDipp1 at varying pH values, for 20 min, at 37 °C. At pH 4.6 used for crystallization of the Dipp1/IP7 complexes (noted by an asterisk [*]), no Dipp1 phosphatase activity was observed. (B) Structural determination of Vip1 and IP6K IP7 products at near 1.2-Å resolution. Vip1 product may be unambiguously defined as D-1-IP7 as the protein complex has left-handed α-helices and the pyrophosphate density is clearly one-carbon clockwise of the D-2 axial phosphate. The product of the IP6K product is defined as D-5-IP7. Difference density contoured at 3σ (shown in green) represents Fo − Fc difference Fourier maps using phases calculated from the final model with the ligand omitted (composite omit).