Figure 5.
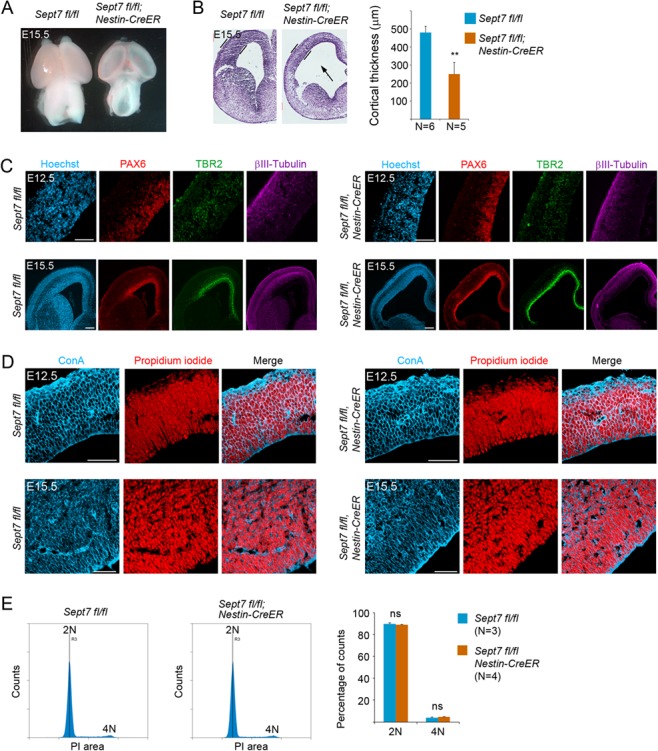
Inducible knockout of Sept7 in NPCs causes growth retardation of the brain without affecting cytokinesis. (A) Tamoxifen was administered to pregnant mother at E9.5 and E10.5 consecutively, and embryonic brains were collected at E15.5 for analyses. At this stage of development, Nestin-CreERT2; Sept7fl/fl homozygous mutant brains showed smaller size and more transparent cortical hemispheres than the control littermate brains. (B) Nissl staining of brain sections revealed thinner cortices of the Nestin-CreERT2; Sept7fl/fl homozygous mutant brains at E15.5. The arrow indicates enlarged ventricle in the mutant brains. **P < 0.01 (Student’s t-test). (C) Cellular marker staining revealed an overall reduction in the thickness of cell ribbons representing radial glial cells (PAX6+), intermediate progenitor cells (TBR2+), and neurons (βIII-Tubulin+) in the cortices of Sept7 inducible knockout mice compared with the wild-type littermate brains. Scale bars represent 100 μm. (D) No obvious bi- or multinucleated cells were seen in the wild-type or Sept7 inducible knockout mutant cortices at E12.5 or E15.5. ConA (blue) and PI (red) were used for visualizing cell peripherals and nuclei, respectively. Scale bars represent 100 μm. (E) Cell cycle analysis of wild-type and mutant cortical cells. Tamoxifen was administered to Sept7 inducible knockout mice at E9.5 and E10.5 consecutively. Littermates of Nestin-CreERT2; Sept7fl/fl and control Sept7fl/fl embryos were collected at E15.5. Cortical cells were examined for their DNA contents by FACS analysis. 2 N, cells in G1/S phase; 4 N, cells in G2/M phase or binucleated cells; ns, not significant (P > 0.05).
