Figure 7.
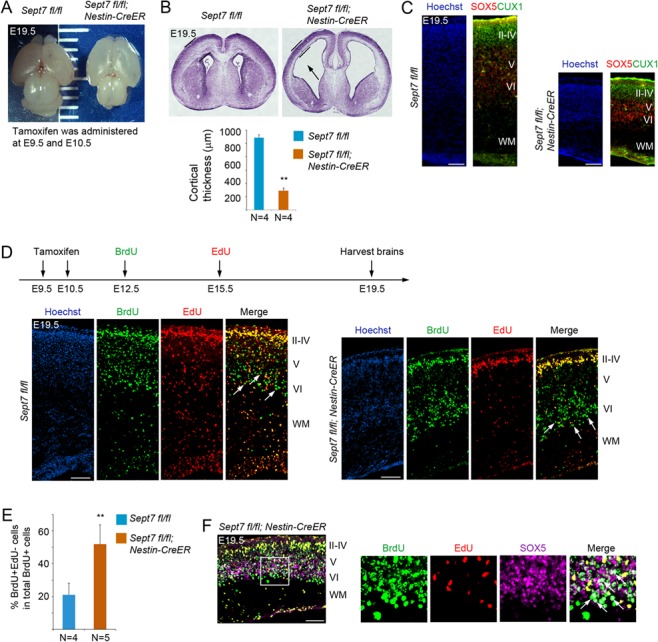
Inducible knockout of Sept7 in NPCs causes ventriculomegaly and defect in neuronal production. (A) Tamoxifen was administered to Sept7 inducible knockout mice at E9.5 and E10.5 consecutively. Pups were delivered by C-section at E19.5. Inducible knockout of Sept7 yielded brains with elongated cortical hemispheres. (B) Sections of Nestin-CreERT2; Sept7fl/fl homozygous mutant brains revealed thinner cortices and dilated ventricles (black arrow). **P < 0.01 (Student’s t-test). Error bars represent SD. (C) Cellular marker staining showed that early-born neurons (SOX5+) and late-born neurons (CUX1+) were correctly located in the respective deep and upper layers in the cortices of Sept7 knockout mice, suggesting that LOF of SEPT7 did not affect cortical lamination. Scale bars represent 100 μm. (D) Tamoxifen was administered to Sept7 inducible knockout mice at E9.5 and E10.5 consecutively. BrdU and EdU were sequentially administered at E12.5 and E15.5, respectively. In Sept7 knockout mice, BrdU+EdU− cells (early-born neurons) and BrdU+EdU+ cells (late-born neurons) were properly located in the respective deep and upper layers. Relatively more BrdU+EdU− cells within the BrdU+ cell population could be seen in the deep layers of Sept7 mutant brains compared with wild-type littermates, reflecting early cell cycle exit due to LOF of SEPT7. Arrows indicate examples of BrdU+EdU− cells. Scale bars represent 100 μm. (E) Quantification of panel D. **P < 0.01 (Student’s t-test). Error bars represent SD. (F) Many BrdU+EdU− cells in the deep layers (boxed area) of Sept7 mutant cortices were positive for SOX5, indicating that these cells have exited cell cycle and differentiated into neurons (early-born neurons). Arrows indicated examples of BrdU+EdU−SOX5+ cells. Scale bar represents 100 μm.
