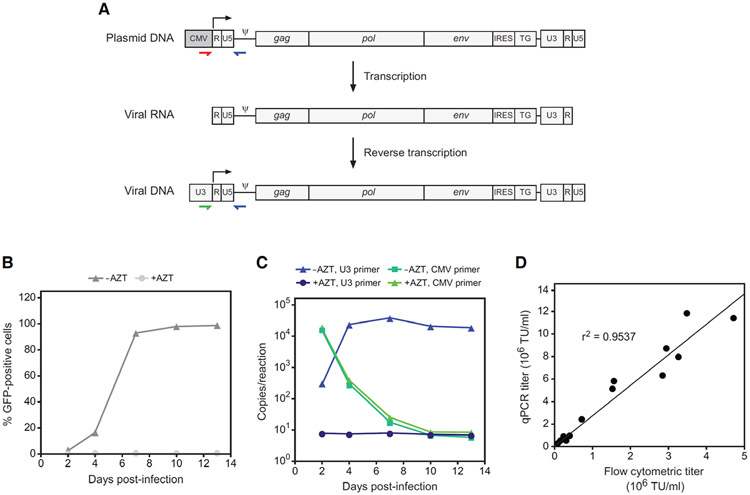
Figure 1.
qPCR assay for determining provirus copy number and biological titer of RRV. (A) Strategy for detection of virus versus RRV plasmid contaminants. The stepwise changes in the RRV genome that occur through transcription of the plasmid and reverse transcription of the viral RNA are shown. Colored arrows indicate the binding location of primers that specifically amplify either RRV plasmid or virus. The use of a CMV-specific forward primer (red) will detect RRV plasmid in infected cells, either carried over from transfection or from another source, whereas a U3-specific primer (green) will detect only genuine reverse-transcribed RRV genomes. (B) RRV-mediated transmission of GFP expression following infection of cultured cells at low MOI with virus produced by transient transfection. In control cultures, AZT was included in the medium from the time of infection. GFP was detected by flow cytometry at 2, 4, 7, 10 and 13 days post-infection. (C) PCR quantitation of virus versus plasmid copies in genomic DNA from the same cultures as in panel B. The reactions employed a forward primer specific either for MLV U3 or for the CMV promoter. Note that the copy numbers per reaction below 50 are outside of the linear range of the assay. (D) Correlation between titer as determined by copy number determination by qPCR versus analysis of GFP expression by flow cytometry.