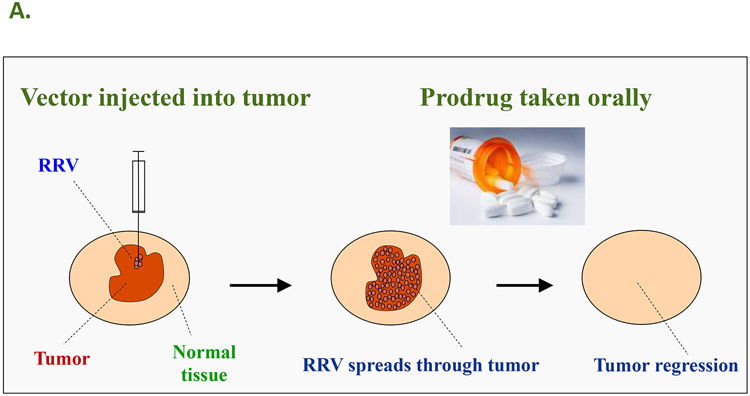
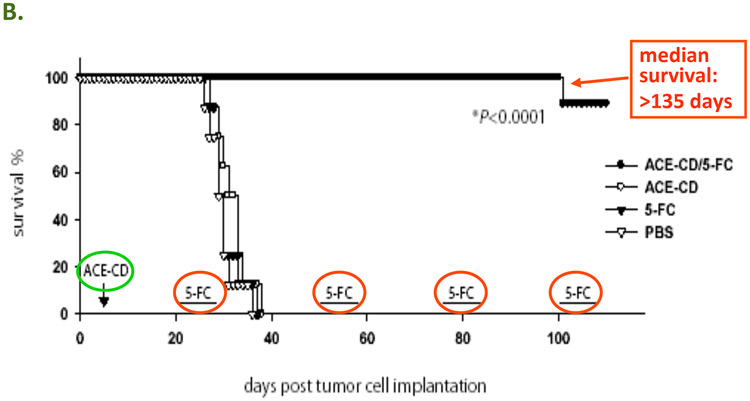
Figure 4.
A: The concept of Controlled Active Gene Transfer: Vector spreads through tumor but not normal tissue, hence the prodrug is activated into an anti-cancer drug only in the infected tumor cells. In the case of human clinical trials currently being conducted by Tocagen Inc., the RRV contains the cytosine deaminase (CD) gene from yeast. This enzyme converts the prodrug 5-FC, an FDA-approved anti-fungal compound that can be taken as an oral pill, into the anti-cancer drug 5-FU directly within the RRV-infected cancer cells. B: RRV-mediated prodrug activator gene therapy can achieve long-term survival in a U-87 intracranial glioma model. PBS: Control group injected with phosphate-buffered saline instead of vector and prodrug. 5-FC: Control group injected with PBS instead of RRV, followed by systemic prodrug administration. ACE-CD: Control group injected with RRV expressing CD gene, but without receiving prodrug afterward. ACE-CD/5-FC: Treatment group receiving both RRV and prodrug. In the treatment group, only a single injection of vector was performed (green circle), followed by multiple cycles of prodrug (red circle).