SUMMARY
Background:
Sepsis is a life-threatening condition often manifested as marked inflammation and severe coagulopathy. Toll-like receptors (TLRs) play a pivotal role in inflammation, organ dysfunction, and mortality in animal sepsis.
Objectives:
Here we investigated the role of TLR signaling in mediating sepsis-induced coagulopathy (SIC) in a mouse model.
Methods:
Polymicrobial sepsis was created by cecal ligation and puncture (CLP) or fecal slurry peritoneal injection. To quantify global clotting function, two viscoelastic assays were performed using rotational thromboelastometry (ROTEM) and presented as maximum clot firmness (MCF): 1) EXTEM to test tissue factor (TF)-initiated clot formation and 2) FIBTEM to test EXTEM in the presence of a platelet inhibitor, cytochalasin D. Plasma coagulation factors were quantified by ELISA. TF gene and protein expression was determined by qRT-PCR and flow cytometry, respectively.
Results:
Between 4 and 24 hours after CLP surgery, WT mice exhibited significant MCF reduction in both EXTEM and FIBTEM tests. This was accompanied by a marked thrombocytopenia and a significant increase in plasminogen activator inhibitor-1, plasma TF, and d-dimer. In comparison, TLR2−/− and TLR7−/− CLP mice exhibited preserved MCF, platelet counts, and near normal plasma TF concentration. Bone marrow-derived macrophages treated with TLR2 agonist (Pam3cys) or TLR7 agonist (R837) had a marked increase in TF gene and protein expression. microRNA-146a, a newly identified proinflammatory mediator upregulated during sepsis, induced TF production via a TLR7-dependent mechanism.
Conclusions:
Murine sepsis leads to increased procoagulant response, thrombocytopenia, and global coagulopathy. TLR2 and TLR7 plays an important role in procoagulant production and in SIC.
Keywords: sepsis, coagulopathy, ROTEM, Toll-like receptor, microRNA
INTRODUCTION
Sepsis is a clinical syndrome caused by a dysregulated host inflammatory response secondary to infection (1) and often characterized by marked systemic inflammation and coagulation activation, the two key components inextricably linked in sepsis leading to gross clotting dysfunction, a condition termed sepsis-induced coagulopathy (SIC) (2–6).
Under normal condition, tissue factor (TF), a potent initiator of the extrinsic coagulation cascade, binds factor VII to form the tenase complex leading to factor X activation, thrombin and fibrin generation, and thrombus development (7). Antithrombin III (ATIII) is a potent inhibitor of activated factor X and thrombin and functions to prevent further activation of coagulant proteases. Effective clot resolution is achieved by activation of the fibrinolytic system and balanced by plasminogen activator inhibitor-1 (PAI-1) (8, 9). In sepsis, TF production is markedly increased by intravascular cells under inflammatory conditions and consequently leads to a profound increase in systemic procoagulant activities (10–12). Persistent procoagulant activation may lead to exhaustion of clotting factors and platelets, and development of a consumptive coagulopathy (3). Further impacting this is reduced ATIII, and inefficient removal of fibrin deposition due to suppression of fibrinolysis by PAI-1 (13, 14).
Toll-like receptors (TLRs), an essential part of host innate immunity, play a pivotal role in initial pathogen recognition and subsequent inflammatory responses. TLRs can also sense host endogenous molecules known as danger-associated molecular patterns or DAMPs (5). We have recently discovered that significant amount of host cellular miRNAs and extracellular vesicles are released into the blood circulation in sepsis and that certain extracellular (ex) miRNAs including miR-146a-5p can function as DAMPs and drive inflammation via a TLR7-dependent pathway (15–18). Finally, stimulation of TLR2 induces PAI-1 expression and increased fibrin deposition in lung microvasculature (19) and bacterial DNA (CpG)-activated TLR9 signaling induces TF expression in endothelial cells (20).
In this study, we tested the hypothesis that TLRs play a pivotal role in host procoagulant responses and contribute to SIC. Using rotational thromboelastometry (ROTEM) (21, 22), we examined the role of various TLR signaling in SIC, explored the underlying mechanisms, and tested the effect of TLR activation in procoagulant expression in immune cells.
MATERIALS AND METHODS
Reagents
All reagents used in ROTEM assays were purchased from Instrumentation Laboratory (TEM® Innovations, Munich, Germany). ELISA kits for fibrinogen and ATIII were from Innov-Research (Novi, MI), TF, interleukin-6, and PAI-1, and PE-conjugated TF antibody were from R&D (Minneapolis, MN), and d-dimer from Diagnostic Stago (Parsippany, NJ). Pam3cys-Ser-(Lys)4 (Pam3cys) was from Enzo Life Science (Farmingdale, NY), Imiquimod (R837) was from Invivogen (San Diego, CA). miScript II RT Kit and the miScript SYBR Green PCR Kit were purchased from QIAGEN (Valencia, CA). PCR primers and miRNA mimics were made by IDT (Coralville, IA).
Animals
All experiments were performed in 8–16-week old male mice with the approval of the Institutional Animal Use and Care Committee of the University of Maryland. WT (C57BL/6) and all knock-out mice used in the study were purchased from the Jackson Laboratory (Bar Harbor, ME) and previously crossed into the C57BL/6 strain. All mice were fed pathogen-free diet. Myd88−/− mice had access to special antibiotic water containing sulfamethoxazole USP 200 mg (40 mg/ml) and trimethoprim USP 40 mg (8 mg/ml) which was stopped 2 weeks prior to experiments. Mice were housed under pathogen free and temperature-controlled conditions with 12-hour light/dark cycles.
Cecal ligation and puncture model of polymicrobial sepsis
Polymicrobial sepsis was created by cecal ligation and puncture (CLP) as previously described (16, 18). Operator (BW) was blinded to the animal strain and group information. In brief, following induction of anesthesia with ketamine 100 mg/kg and xylazine 4 mg/kg solution, CLP was performed via laparotomy. Ligation was performed 1.5 cm from the distal tip of the cecum ensuring that the ligation point was distal to the ileocecal valve. A through and through puncture with an 18-gauge needle was made mid cecum and 2–3 mm of feces was extruded. The cecum was then placed back into the peritoneal cavity and the peritoneum and skin closed. Sham control mice underwent a laparotomy only without CLP. Prewarmed normal saline (0.03 mL/g) was administered subcutaneously (SQ) at the end of each procedure for fluid resuscitation, and bupivacaine (3 mg/kg) and buprenorphine (0.1 mg/kg) were administrated SQ for postoperative pain control.
Fecal Slurry (FS) model of Polymicrobial Sepsis
Gender and age matched WT and TLR7−/− mice were euthanized via cervical dislocation after induction of anesthesia with ketamine 100 mg/kg and xylazine 4 mg/kg solution. The entire cecum was dissected and removed. Cecum fecal contents were collected, weighed, and then resuspended in 5% dextrose-water (D5W) solution (Hospira, Lake Forest, IL) at a final concentration of 80 mg/mL. After thorough mixing, the fecal slurry was then filtered through a 100-μm cell strainer. WT mice were injected i.p. with fecal slurry solution at 1.3 mg/g body weight in a volume of 500 μL. The control group received an equivalent volume of D5W intraperitoneally. The two operators (LZ and BW) were blinded to strain group information.
Blood collection
Whole blood was collected via cardiac puncture under general anesthesia and transferred to 1 mL vacuette capillary blood collection tubes containing 3.2% sodium citrate for complete blood count using an automated cell counter and for rotational thromboelastometry (ROTEM; TEM® Innovations, Gmbh, Munich, Germany) experiments. Platelet poor plasma was prepared from the remaining blood samples and used for future ELISA analysis.
Hemostatic viscoelastic testing
EXTEM and FIBTEM assays were performed in whole blood using mini-cups that contain 7 μL 0.2 M CaCl2 and 7 μL TF (for EXTEM) or 7 μL 0.2 M CaCl2, 7 μL TF, and cytochalasin D (for FIBTEM), and the whole blood samples were warmed to 37°C for 5 minutes. Maximum clot firmness (MCF) was recorded in whole blood collected at the indicated time points following sham or CLP procedure or at 20 hours following fecal slurry injection.
Tissue histology
Liver tissues were collected from mice at 24 hours following sham or CLP surgery and subsequently fixed in formalin followed by 70% ethanol. Tissue samples were paraffin embedded and sectioned onto slides and stained with hematoxylin-and-eosin (H&E). Liver sections were read by an independent pathologist.
ELISA
Plasma was prepared at room temperature and stored at −80 °C. ELISA kits were used to determine plasma levels of fibrinogen, ATIII, tissue factor, PAI-1, d-dimer and interleukin-6 at the indicated time points.
Mouse bone marrow derived macrophages (BMDMs)
BMDMs were isolated from mice and cultured as previously described (23).
Treatment of cell cultures
Macrophage cultures were allowed to reach 80% confluence. Cells were washed twice and incubated in RPMI-1640 with 0.05% BSA for 1-hour before treatment. miRNAs were complexed with lipofectamine 3000 (ThermoFisher Scientific, Waltham, MA) and incubated for 15 minutes at room temperature prior to cell treatment.
RNA extraction and RT-qPCR
RNA was extracted from cells with TRIzol and quantified using NanoDrop. One μg/ml RNA was used for subsequent cDNA transcription using the miScript II RT kit. Quantitative PCR was performed using the miScript SYBR Green PCR kit. Fold difference in transcript expression among samples was calculated as relative gene expression normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the relative Ct method. The specific primers used for each PCR experiment are listed below:
GAPDH: Forward: 5’-AACTTTGGCATTGTGGAAGG-3’, Reverse: 5’-GGATGCAGGGATGATGTTCT-3’; Tissue factor: Forward primer: 5’-AGGATGTGACCTGGGCCTAT-3’, Reverse primer: 5’-GGCTGTCCAAGGTTTGTGTC-3’.
Flow Cytometry analysis of TF expression
BMDMs were detached and harvested using 0.25% Trypsin/EDTA followed by centrifugation. Cells were washed with cold DPBS once and incubated with 50 μL of anti-mouse TF-PE antibody for 30 minutes on ice. The cells were washed and resuspended in DPBS with 5% FBS and analyzed in a BD FACS Canto flow cytometer (BD Biosciences). Unstained cells were included as negative control. The number of cells positive for TF expression was expressed as percentage of total loaded cells.
Statistical analysis
Statistical analysis was performed using Prism 7 software (GraphPad, La Jolla CA). For measurements below detection limit, values at the lowest detection limit were used. Correlations were conducted using Pearson’s test with 95% confidence bands. Parametric data were analyzed by two-tailed unpaired students t-test with Welch’s correction if unequal sample size or variance was present for intra-group comparison and 1-way Anova with Bonferroni’s test for multiple comparisons to a reference standard (WT CLP). Data are expressed as mean ± SD or SEM as indicated. P values less than 0.05 were considered statistically significant.
RESULTS
Septic mice develop global coagulopathy
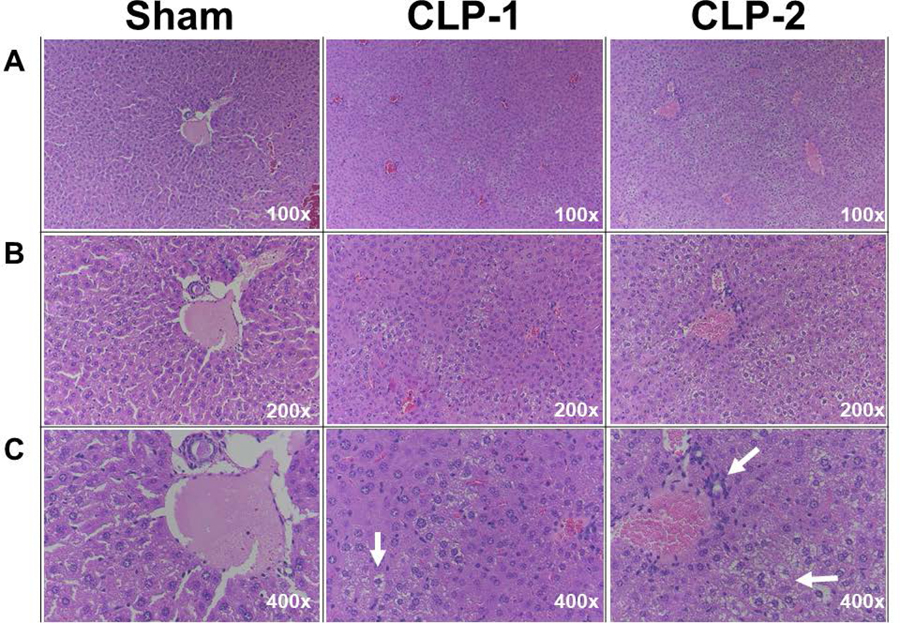
CLP mice exhibited a time-dependent hypothermia (rectal temperature; 4 h: 30.5±0.5 °C, 8 h: 29.8±0.7 °C, 24 h: 28.0±0.6 °C vs. sham, 24 h: 35.3±0.3 °C). CLP mice also had lower circulating white blood cells (1.2±0.17 x 103/μL vs. 4.3±0.79 x103/μL, P=0.0002). Moreover, hepatic histological revealed evidence of widespread microvesicular steatosis, which is a common finding in sepsis and indicates early hepatic dysfunction (24), but lack of microvascular thrombosis (Fig. 1).
Figure 1. Hepatic injury of CLP mice.
Twenty-four hours after sham or CLP surgery, liver tissue was collected, fixed in formalin for 48 hours, followed by 70% ethanol. Liver sections were stained with H&E staining and assessed by a pathologist. Hepatic microvesicular steatosis and vacuolization are indicated by the white arrows in the CLP samples.
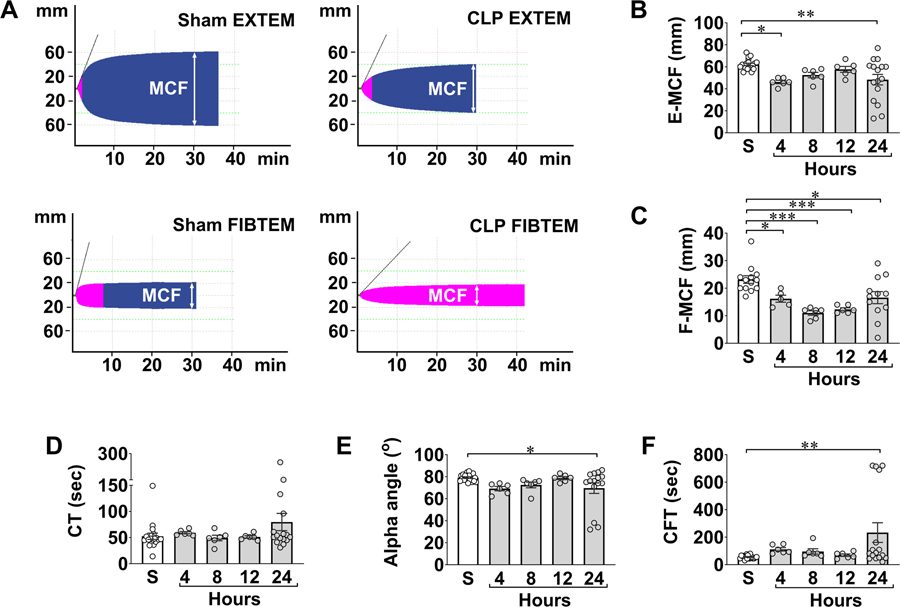
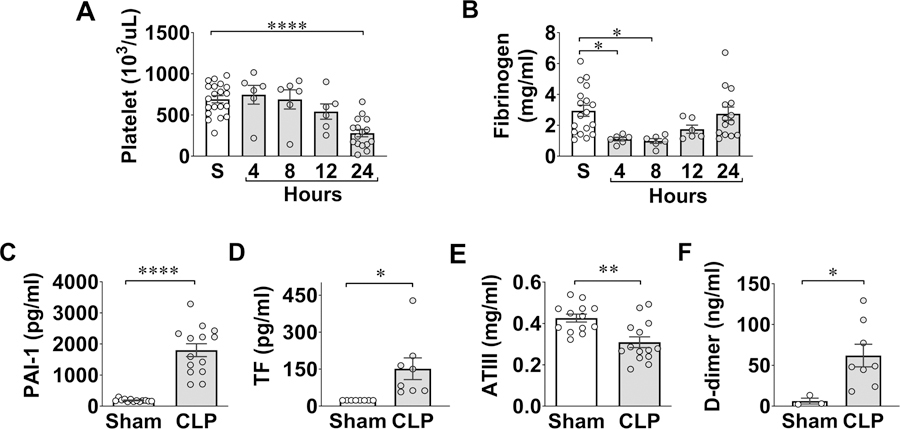
Fig. 2A is representative EXTEM and FIBTEM tracings in sham and CLP mice with MCF marked. The accumulated data in Fig. 2B–C demonstrated that CLP mice developed time-dependent global coagulopathy as evidenced by an early reduction in MCF at 4 hours followed by sustained reductions of E-MCF/F-MCF for up to 24 hours. Other ROTEM parameters, such as clot formation time (CFT) and α-angle, which indicate rate and strength of fibrin-platelet polymerization, were prolonged or reduced, respectively, at 24 hours in CLP mice (Fig. 2 D–F). Importantly, the clotting dysfunction corresponded to progressive declines in platelet count that reached lowest level at 24 hours (Fig. 3A). Plasma fibrinogen levels dropped early within 4–8 hours and rebounded somewhat at 24 hours (Fig. 3B).
Figure 2. Septic mice develop global coagulopathy.
WT C57BL/6 mice were subjected to CLP surgery and sacrificed at indicated time points for blood collection. Sham mice were sacrificed at 24 hours. A, Representative pictures of ROTEM tracings. Both representative EXTEM and FIBTEM tracings from sham and CLP mice are shown. MCF is marked in each tracing at 30 min. B-C, Time course of MCF values in EXTEM and FIBTEM assays following CLP. *P<0.05, **P<0.01, ***P<0.001. D-F, Time course of clotting time (CT), alpha angle, and clotting formation time (CFT) following CLP procedure. CLP = cecal ligation and puncture, MCF = maximum clot firmness, TLR = Toll-like receptor.
Figure 3. Clotting factors in sham and septic mice.
Plasma was prepared at 24 hours after sham and CLP procedures. A-F, Platelets, plasma fibrinogen, PAI-1, TF, ATIII, and D-dimer were measured as indicated. *P<0.05, **P<0.01, ****P<0.0001. CLP = cecal ligation and puncture, PAI-1 = plasminogen activator inhibitor-1, TF = tissue factor, ATIII = antithrombin III.
To determine the mechanisms underlying SIC, we examined a few plasma proteins known for their key roles in clotting regulation including PAI-1, TF, and ATIII. We found a marked increase in plasma PAI-1 and soluble fraction of TF, two procoagulants proteins, and marked reduction in ATIII, a thrombin inhibitor, in CLP mice (Fig. 3C–E). There was also a marked increase in the plasma d-dimer, a fibrin degradation product, in CLP mice (Fig. 3F). Taken together, these data demonstrate severe global coagulopathy, marked upregulation in procoagulant responses and decrease in anticoagulant factors, and increased coagulation consumption in septic mice.
Role of TLR signaling in sepsis-induced coagulopathy
To test the role of innate immune signaling in SIC, we took a loss-of-function approach and subjected WT mice and mice genetically deficient of various TLRs or their signaling adaptors to sham or CLP procedures. As anticipated, WT mice became severely hypothermic 24 hours after CLP with a marked decrease in rectal temperature (sham: 36.9±0.2 °C vs. CLP: 29.1±0.3 °C, n=33, P<0.0001). Similarly, both TLR3−/− and TLR4−/− CLP mice developed severe hypothermia with the rectal temperatures at 28.7±1.7 °C (P<0.001) and 25.6±0.46 °C (P= 0.01), respectively. In comparison, TLR2−/−, TLR7−/−, MyD88−/−, and Trif−/− CLP mice were less hypothermic (TLR2−/− 32±7 °C, n=6, P< 0.01; TLR7−/− 34±0.9 °C, n=12, p<0.0001, MyD88−/− 34±0.8 °C, n=13, P< 0.0001, Trif−/− 31.2±0.6, P= 0.02), suggesting overall decreased clinical severity in these septic mice.
Compared to WT mice, TLR2−/−, TLR7−/− and MyD88−/− mice exhibited a significant improved coagulation function with preserved E-MCF and F-MCF, whereas TLR3−/− and TLR4−/− CLP mice displayed coagulation impairment with significantly reduced E-MCF/F-MCF similar to WT CLP mice (Table 1). Of note, Trif−/− mice had slightly lower MCF compared to other strains after sham surgery, and exhibited the same MCF between sham and CLP groups, and similar levels of MCF as WT CLP group. Moreover, accompanying the global clotting dysfunction in WT CLP mice was marked reduction in platelet counts (Table 1). In comparison, TLR2−/− and TLR7−/− mice exhibited very mild reductions in platelet counts that were statistically indifferent from their sham counterparts, whereas TLR3−/−, TLR4−/−, MyD88−/− and Trif−/− mice had significant reductions in platelet counts compared to the sham (Table 1). Together, these data suggest that mice lacking TLR2 and TLR7 have better preserved global clotting function and maintained platelet counts at 24 hours during sepsis.
Table 1.
Toll-like receptor deficiency impacts global coagulation dysfunction in sepsis.
| Strain | Intervention | EXTEM | FIBTEM | Platelets |
|---|---|---|---|---|
| MCF (mm) | MCF (mm) | (103/μL) | ||
| WT | Sham | 73.6±0.99 | 35.0±1.46 | 977±34.3 |
| CLP | 58.5±3.03*** | 24.4±1.81*** | 532±51.4**** | |
| TLR7−/− | Sham | 75.3±1.79 | 36.2±2.83 | 953±51.7 |
| CLP | 75.8±1.99ΔΔΔ | 38.1±3.01ΔΔ | 791±64.9Δ | |
| TLR2−/− | Sham | 76.8±1.01 | 36.0±2.02 | 1126±36.1 |
| CLP | 77.2±1.92ΔΔ | 40.2±2.90ΔΔ | 947±157ΔΔΔΔ | |
| MyD88−/− | Sham | 67.8±1.79 | 28.2±2.75 | 941±37.1 |
| CLP | 72.0±2.57 | 35.1±2.94 | 515±49.4*** | |
| Trif−/− | Sham | 66.3±1.80 | 26.8±1.65 | 1023±51.6 |
| CLP | 64.3±6.20 | 30.8±3.05ΔΔΔΔ | 594±88.7** | |
| TLR3−/− | Sham | 76.7±0.61 | 40.3±1.38 | 1046±40.3 |
| CLP | 62.2±3.16** | 17.8±4.09*** | 571±99.9** | |
| TLR4−/− | Sham | 76.8±2.11 | 36.4±4.94 | 1265±249 |
| CLP | 50.2±5.42** | 18.0±6.49# | 306±70.8** | |
* or # represents significant difference between sham and CLP mice within the same strain.
represents significant difference between WT CLP and TLR−/− CLP mice
P = 0.05
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
Sample sizes are as follows: WT (Sham= 18–23, CLP= 26–33), TLR3−/− (Sham= 5–6, CLP= 5–6), TLR4−/− (Sham= 5, CLP= 4–5), TLR2−/− (Sham= 6, CLP= 6), TLR7−/− (Sham= 7–11, CLP= 7–12), MyD88−/−(Sham= 7–8, CLP= 12–13), and Trif−/− (Sham= 4, CLP= 6)
CLP = cecal ligation and puncture, MCF = maximum clot firmness, TLR = Toll-like receptor.
Impact of TLR deletion on plasma coagulants and cytokine in sepsis
To further explore the mechanistic role of TLR signaling in SIC, we examined plasma TF, fibrinogen, PAI-1, and IL-6 in sham and CLP mice. Similar to WT mice, TLR3−/−, TLR4−/−, and Trif−/− CLP mice had higher plasma concentrations of soluble TF compared to their sham controls (Table 2). In contrast, TLR2−/−, TLR7−/−, and MyD88−/− CLP mice showed much attenuated plasma TF concentrations (Table 2). At 24 hours, the plasma fibrinogen levels were similar between CLP and sham WT mice. TLR3−/− and TLR4−/− CLP mice had slightly lower plasma fibrinogen compared to their sham counterparts, but differences did not reach statistical significance (Table 2). In contrast, TLR2−/−, TLR7−/−, MyD88−/−, and Trif−/− mice all exhibited significant elevations in plasma fibrinogen levels compared sham controls (Table 2). Plasma PAI-1 was nearly undetectable in sham mice but markedly elevated in WT mice 24 hours after CLP, and this increase in PAI-1 remained largely unchanged across all genotypes (Table 2). WT CLP mice also exhibited a marked increase in plasma IL-6, and such cytokine storm was markedly attenuated (> 90%) in TLR2−/−, TLR7−/−, and MyD88−/−, and to a lesser degree in TLR3−/− and Trif−/− mice when compared with WT CLP mice (Table 2).
Table 2.
Toll-like receptor deficiency impacts plasma procoagulants and IL-6 at 24 hours after sham or CLP surgery.
| Strain | Intervention | TF | Fibrinogen | PAI-1 | Il-6 |
|---|---|---|---|---|---|
| (pg/mL) | (mg/mL) | (pg/mL) | (pg/mL) | ||
| WT | Sham | 22.9±0.99 | 3.08±0.13 | 47.6±26.3 | 58.6±14.1 |
| CLP | 112.6±20.7*** | 3.53±0.31 | 39,001±10828** | 142,890±24442**** | |
| TLR7−/− | Sham | 23.4±0 | 2.91±0.12 | 28.1±12.29 | 118±4.25 |
| CLP | 24.7±5.7Δ | 4.41±0.62* | 23,571±7563** | 12,249±3391**/ΔΔΔ | |
| TLR2−/− | Sham | 23.4±0 | 3.05±0.18 | 9.38±0 | 15.6±0 |
| CLP | 39.3±5.97* | 4.04±0.23** | 52,027±12927** | 4,734±3239ΔΔ | |
| MyD88−/− | Sham | 23.4±25 | 2.22±0.27 | 9.38±0 | 51.3±16.6 |
| CLP | 25±4.52Δ | 4.54±0.39*** | 27,206±8099* | 6,814±2009*/ΔΔΔ | |
| Trif−/− | Sham | 23.4±0 | 2.72±0.12 | 9.38±0 | 16.7±5.95 |
| CLP | 68.7±35.3 | 5.88±0.45*** | 46,040±16549* | 64,686±32823 | |
| TLR3−/− | Sham | 23.4±0 | 2.93±0.15 | 9.38±0 | 7.69±3.24 |
| CLP | 92.8±33.8 | 2.36±0.47 | 26,114±6338** | 39,778±16656 | |
| TLR4−/− | Sham | 23.4±0 | 3.26±0.21 | 9.38±0 | 66.4±23.2 |
| CLP | 113.9±30.9* | 2.12±0.60 | 44,391±16723* | 109,463±34771** | |
* or # represents significant difference between sham and CLP mice within the same strain.
represents significant difference between WT CLP and TLR−/− CLP mice.
P = 0.05
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
WT (Sham= 18–23, CLP= 26–33), TLR3−/− (Sham= 5–6, CLP= 5–6), TLR4−/− (Sham= 4–5, CLP= 4–5), TLR2−/− (Sham= 6, CLP= 5–6), TLR7−/− (Sham= 7–11, CLP= 7–12), MyD88−/− (Sham= 7–8, CLP= 8–12), and Trif−/− (Sham= 4, CLP= 6)
CLP = cecal ligation and puncture, MCF = maximum clot firmness, TLR = Toll-like receptor.
We also observed a moderate but significant negative correlation between plasma TF levels and E-MCF (r = −0.5287, P=0.0079), and a positive correlation between plasma TF and IL-6 concentrations (r = 0.447, P=0.0221) (data not shown), supporting the premise of inflammation-associated consumptive coagulopathy in polymicrobial sepsis. Together, these data suggest that innate immune signaling via TLR2 and TLR7 plays an important role in mediating coagulation activation and systemic inflammation during sepsis.
Impact of WT and TLR7−/− fecal slurry injection on global coagulopathy in WT mice
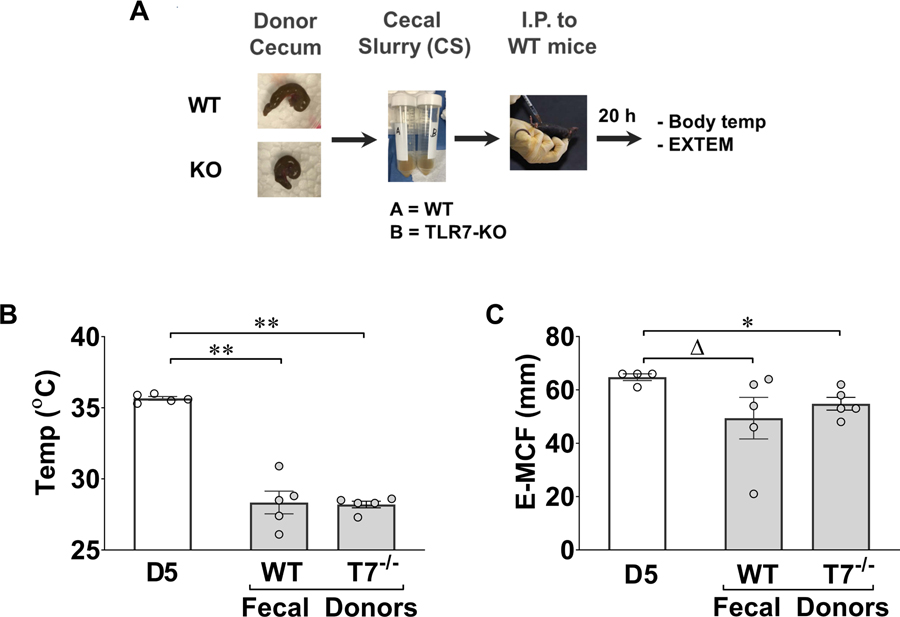
The gut microbiome is affected by both environment and host genotype (25). Previous studies, however, have shown TLR2 genetic deletion does not change intestine bacterial composition (26). Thus, we decided to test virulence of the gut microbiome from WT and TLR7−/− mice in a fecal slurry model (Fig. 4A). In these experiments, we injected the same amount of fecal slurry (1.3 mg/g body weight) from WT and TLR7−/− mice into the peritoneal cavity of WT mice. Twenty hours later, we measured rectal temperature and global clotting function. As shown in Fig. 4B–C, both WT and TLR7−/− fecal slurry injection resulted in similar degrees of hypothermia and E-MCF reduction.
Figure 4. Fecal slurry (FS) model of polymicrobial sepsis.
A. Diagram of the fecal slurry peritonitis model. B. Core temperature. Fecal slurry was collected from WT, TLR7−/−, and TLR2−/− mice and injected i.p. to WT mice. Control mice received i.p. injection of 5% dextrose in water (D5). Twenty-four hours later, rectal temperature was measured. C. EXTEM assay. Blood was collected for E-MCF assay. ΔP = 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.
Activation of TLR2 and TLR7 induces procoagulant production in BMDMs
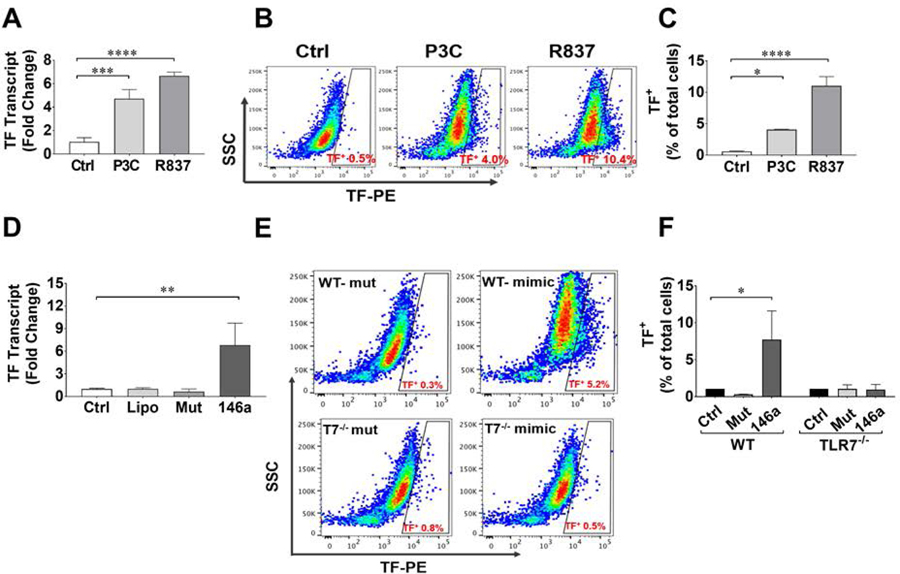
To further determine the role of TLR2 and TLR7 in coagulation activation, we tested whether or not activation of TLR2 or TLR7 signaling is capable of inducing TF expression. We treated BMDMs isolated from WT mice with specific agonists for TLR2 (Pam3Cys) and TLR7 (R837) and measured TF mRNA by qRT-PCR (Fig. 5A) and cell surface expression by flow cytometry (Fig. 5B). Pam3Cys and R837 induced 4–6-fold increase in TF mRNA expression (Fig. 5A) and a significant increase in the percentage of cells positive for surface TF protein (Fig. 5C).
Figure 5. Toll-like receptor (TLR) ligands and miR-146a induce tissue factor (TF) production in mouse macrophages via TLR7-dependent mechanism.
A. TF mRNA production in BMDMs treated with TLR2 agonist, Pam3Cys (P3C) 10 μg/ml and TLR7 agonist, Imiquimod (R837) 1 μg/ml for 4 hours. n = 3/group. B and C. Cell surface expression of TF in BMDMs. Cells were treated with P3C (10 μg/ml) and R837 (1 μg/ml) for 16 hours. Cells were gated for TF using flow cytometry (B) and expressed as % of total cells (C). N=4/group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. D. Cells were stimulated with single-stranded miR-146a-5p mimic or its U→A mutant at 50 nM for 4 hours. miRNA mimics were complexed with lipofectmaine 3000 prior to transfection. N=3/group. **P<0.01. E-F. TF expression as measured by flow cytometry. WT or TLR7−/− BMDMs were treated with miR-146a and its U→A mutant at 50 nM for 16 hours. N=3/group. *P<0.05. BMDM = bone marrow derived macrophages. TF = tissue factor. TLR = Toll-like receptor
TLR7 is a sensor for single-stranded RNA virus (27) and plays a pivotal role in inflammation (18). We have found that plasma miRNAs such as miR-146a-5p are elevated following polymicrobial infection (16). To test if miRNA is capable of activating coagulant production, we challenged WT and TLR7−/− BMDMs with miR-146a or its mutant. As shown in Fig. 5D, exogenous miR-146a-5p mimic, but not its U→A mutant, induced a significant increase in TF gene expression. It also resulted in TF protein expression in WT, but not TLR7−/−, BMDMs (7.7±3.9% vs. 0.3±0.02%) (Fig. 5E–F).
DISCUSSION
We have established a murine sepsis model that is featured by hypothermia, cytokine storm, and coagulation dysfunction. WT CLP mice exhibited reduced coagulation function and severe thrombocytopenia within 24 hours. Thrombotic formation and platelet aggregation are the possible cause of the drop of platelet count. However, other causes such as hypothermia can affect circulating platelet number and function in vivo (28–30). Mice subjected to 4 °C temperature have a time-dependent decrease in circulating platelets up to 22% (30). Thus, hypothermia may in part contribute to the decrease in circulating platelets, but it alone would not fully explain the 57% decrease in platelet counts we observed in these septic mice. It is likely that a combination of low temperature, platelet-leukocyte aggregates, and clotting consumption in sepsis lead to the marked thrombocytopenia, which would contribute to the subsequent declines of E-MCF and sepsis-induced coagulopathy.
There was a significant corresponding reduction in both fibrinogen and F-MCF within 4–8 hours after CLP. Fibrinogen levels increased over time between 8–24 hours with concurrent increases in the F-MCF values. It is worth noting that FIBTEM assesses both quantitative and qualitative fibrin function and is sensitive to changes in factor XIII levels, which is primarily responsible for covalent crosslinking of fibrins and stabilizing the formed clot (31). We have noticed that F-MCF in septic mice remained significantly lower than sham controls at 24 hours despite rebound fibrinogen levels. We speculate that the F-MCF results may be more evident of fibrin polymerization impairment secondary to weak fibrin interactions or factor XIII activity rather than reduced fibrinogen quantity (32).
We noted that septic mice had elevated procoagulant proteins including PAI-1 and TF. Cytokines and upregulated PAI-1 and TF could lead to persistent thrombin generation, platelet activation, and thrombotic burden, a hypercoaguable condition as reported previously in a mouse model of acinetobacter baumannii infection (33). However, we only observed a clotting profile consistent with hypocoagulability. The increase in plasma TF and PAI-1 levels and concurrent decrease in plasma ATIII and elevated D-dimer suggest an activated coagulation process with reduction in clot quality (i.e., decreased MCF) and consumption of platelets (34).
Previous studies show that polymicrobial sepsis enhances photochemical-induced thrombosis in a TLR2-dependent manner (35). Hellman, et al. have reported that treatment with TLR2 agonists leads to increased TF production in HUVECs (19). Consistent with these earlier reports, we found that septic TLR2−/− mice had markedly reduced plasma IL-6, attenuated TF levels, higher platelet counts, and improved global coagulation compared with septic WT mice. Moreover, TLR2 agonist was able to induce TF synthesis and cell surface expression. Together, these data suggest a pivotal role of TLR2 in mediating the procoagulant response and subsequent coagulopathy during polymicrobial sepsis.
We have demonstrated that cellular RNA and certain uridine-rich miRNA mimics are highly proinflammatory and capable of stimulating the innate immune response via TLR7 and MyD88 signaling pathway both in vitro and in vivo (15–17). Our findings that TLR7−/− CLP mice had improved MCF on ROTEM, higher platelet counts and fibrinogen levels, and attenuated cytokine and TF production supports the hypothesis that TLR7 signaling mediates proinflammatory and procoagulant responses in sepsis. This is further supported by our in vitro observations. Both R837 and exogenous miR-146a-5p induced significant TF expression via a TLR7-dependent mechanism. These findings establish a novel role for TLR7 as an important player in the development of sepsis-induced coagulopathy.
MyD88−/− and Trif−/− mice exhibited a significant thrombocytopenia, yet have well preserved MCF in both EXTEM and FIBTEM. We speculate that the significantly higher level of fibrinogen in both MyD88−/− and Trif−/− septic mice was able to compensate for the reduction in platelet counts, thereby resulting in preserved MCF. This underscores the greater impact of the plasma fibrinogen levels on MCF and the importance of fibrin to clot formation in the face of ongoing coagulation activation.
Platelets, traditionally associated with hemostasis and thrombus formation, also plays an active role in innate immune system. Similar to other immune cells, platelets can react to specific pathogen and host-derived molecules, known as PAMPs and DAMPs, respectively, via surface and intracellular TLRs. For example, in response to bacterial lipoproteins, platelets show a distinct pattern of alpha granule release and increased platelet monocyte aggregates in vivo (36). Activation of platelet TLR7 signaling by the agonist loxorobine in vivo also causes an early and sustained reduction in platelet counts up to 24 hours attributed to formation of platelet-leukocyte aggregates and internalization of platelet fragments by neutrophils (37). Thus, we speculate that thrombocytopenia observed in CLP mice is secondary to both increase in thrombin-induced platelet-aggregation and interaction with leukocytes. This effect appears to be diminished in TLR2−/− and TLR7−/− septic mice.
Fibrinogen is an acute phase reactant and upregulated during acute inflammation. The plasma levels fibrinogen represents a balance between de novo synthesis and consumption. Higher plasma fibrinogen levels in TLR2−/− and TLR7−/− septic mice may reflect the fact these mice have lower inflammation and thrombin generation, and thus less consumption of fibrinogen. Further, higher plasma fibrinogen likely compensates for platelet reductions and thereby maintains E-MCF in TLR2−/−, TLR7−/−, MyD88−/−, and Trif−/− septic mice, thus underscoring the importance of fibrin to clot formation and perhaps greater resistance to consumption during polymicrobial sepsis. A similar observation has been made in human studies of sepsis and ROTEM suggesting fibrinogen has a much greater impact on MCF than changes in platelet count (38).
D-dimer is a marker of fibrinolysis and produced from the fibrinolytic cleavage of polymerized fibrin. D-dimer is reportedly elevated in septic patients and in our study, markedly elevated in WT CLP mice. While we observed an elevation in D-dimer in WT septic mice, we did not see a difference in the plasma D-dimer levels between WT and TLR2−/−, TLR7−/−, or MyD88−/− septic mice (Suppl. Fig. 1). It is clear there was ongoing coagulation activation and enhanced fibrinolysis as evidenced by elevated plasma TF, fibrinogen, and D-dimers, respectively, in septic WT mice. However, in the face of significant elevations of plasma PAI-1, a serine protease inhibitor that functions as the principal inhibitor of tissue plasminogen activator and urokinase, D-dimer levels alone may not accurately reflect the dynamic changes in coagulant consumption among septic WT and TLR-deficient mice because of the ongoing fibrinolytic suppression (13). In future studies, quantifying actual thrombin generation and characterizing the natural progression of sepsis-induced coagulation activation to the late fibrinolytic-DIC stage may help to further clarify the impact of TLR signaling in sepsis-induced coagulopathy.
To rule out the possibility that TLR7 deficiency may alter the gut microbiomes leading to reduced bacterial virulence and improved coagulation profile as compared to WT mice, we tested the virulence of the gut microbiomes in a fecal slurry model. We found that compared with the CLP model, the fecal slurry model generated similar degree of hypothermia (28.0±2.0 °C vs 28.7±1.7°C) and MCF reduction (48.3±19.18 mm vs 52.4±13.28 mm), and comparable levels of plasma cytokines (39) in WT mice. Importantly, we found no difference in the degree of hypothermia and coagulation dysfunction between the WT mice receiving WT vs TLR7−/− fecal slurry. These data suggest that WT and TLR7−/− mouse intestinal microbiome exhibited similar virulence and that any potential difference in microbiomes of WT and TLR7−/− mice may not explain the survival benefit and other protective effects in TLR7−/− CLP mice. This is consistent with a previous study that used fecal bacteria from a single donor and transferred to TLR2−/− and TLR4−/− mice, and found that neither strain had altered the intestinal microbiota composition (26).
We observed hepatic injury in CLP mice as characterized by widespread hepato-steatosis associated with impaired amino acid and fatty acid metabolism (40) and vacuolization of hepatocytes (24). The hepatic injury may be induced by several mechanisms including poor tissue perfusion secondary to severe septic shock and intravascular thrombosis and mitochondrial dysfunction. However, at 24 hours, we did not observe any microvascular thrombosis in the liver. Further study will be needed to detect any microvascular thrombosis formed during the early hypercoagulatory phase of sepsis.
In summary, our studies established a mouse model of sepsis-induced coagulopathy characterized by severely attenuated MCF in ROTEM, increased plasma PAI-1, TF, and IL-6, and markedly decreased platelets and plasma ATIII. Mice deficient of TLR2 and TLR7 had significantly preserved clotting function and reduced procoagulant and cytokine expression under the same conditions. Moreover, we found that TLR2 and TLR7 activation and exogenous miR-146a are sufficient to upregulate tissue factor expression in macrophages. These data suggest that innate immune signaling via TLR2 and TLR7 plays an important role in mediating coagulation activation and sepsis-induced coagulopathy.
Supplementary Material
ESSENTIALS.
Sepsis is associated with circulatory inflammation and severe coagulopathy. Toll-like receptors are known for mediating inflammation in sepsis, but their role in sepsis-induced coagulopathy (SIC) is incompletely understood.
Global clotting function and key clotting factors were measured in WT and mice lacking various TLRs in murine models of SIC.
Septic WT mice developed time-dependent thrombocytopenia and coagulopathy. TLR2- and TLR7-KO mice had improved clotting function. Activation of TLR2 and TLR7 is able to increase tissue factor expression.
TLR2 and TLR7 play an important role in tissue factor production and in SIC.
ACKNOWLEDGEMENTS
We thank Olivia Conn for her excellent technical assistance and Shaheer Hasaan for assistance with training in viscoelastic thromboelastometry testing. We also thank TEM® Innovations for their contribution of MC rod and cup holder mini and MINI cups & pins in support of this project.
Funding support: This work was supported in part by the NIH (Bethesda, Maryland) grants, R01-GM097259/R01-GM122908 (WC), and R35-GM124775 (LZ), Frontiers in Anesthesia Research Award from International Anesthesia Research Society (San Francisco, California) (WC), and the Shock Faculty Research Award from Shock Society (LZ). BW is a recipient of the Seed Funds from the Department of Anesthesiology and from the Center for Shock Trauma Anesthesiology Research.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, and Angus DC. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhainaut JF, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, and Nelson DR. 2005. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med 33: 341–348. [DOI] [PubMed] [Google Scholar]
- 3.Semeraro N, Ammollo CT, Semeraro F, and Colucci M. 2010. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis 2: e2010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delabranche X, Helms J, and Meziani F. 2017. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Poll T, van de Veerdonk FL, Scicluna BP, and Netea MG. 2017. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17: 407–420. [DOI] [PubMed] [Google Scholar]
- 6.Levi M, and Poll T. 2015. Coagulation in patients with severe sepsis. Semin Thromb Hemost 41: 9–15. [DOI] [PubMed] [Google Scholar]
- 7.Grover SP, and Mackman N. 2018. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol 38: 709–725. [DOI] [PubMed] [Google Scholar]
- 8.Rega G, Kaun C, Weiss TW, Demyanets S, Zorn G, Kastl SP, Steiner S, Seidinger D, Kopp CW, Frey M, Roehle R, Maurer G, Huber K, and Wojta J. 2005. Inflammatory cytokines interleukin-6 and oncostatin m induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation 111: 1938–1945. [DOI] [PubMed] [Google Scholar]
- 9.Dong J, Fujii S, Imagawa S, Matsumoto S, Matsushita M, Todo S, Tsutsui H, and Sobel BE. 2007. IL-1 and IL-6 induce hepatocyte plasminogen activator inhibitor-1 expression through independent signaling pathways converging on C/EBPdelta. Am J Physiol Cell Physiol 292: C209–215. [DOI] [PubMed] [Google Scholar]
- 10.Gando S, Nanzaki S, Sasaki S, and Kemmotsu O. 1998. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost 79: 1111–1115. [PubMed] [Google Scholar]
- 11.Xue M, Sun Z, Shao M, Yin J, Deng Z, Zhang J, Xing L, Yang X, Chen B, Dong Z, Han Y, Sun S, Wang Y, Yao C, Chu X, Tong C, and Song Z. 2015. Diagnostic and prognostic utility of tissue factor for severe sepsis and sepsis-induced acute lung injury. J Transl Med 13: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szotowski B, Antoniak S, Poller W, Schultheiss HP, and Rauch U. 2005. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res 96: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 13.Madoiwa S, Nunomiya S, Ono T, Shintani Y, Ohmori T, Mimuro J, and Sakata Y. 2006. Plasminogen activator inhibitor 1 promotes a poor prognosis in sepsis-induced disseminated intravascular coagulation. Int J Hematol 84: 398–405. [DOI] [PubMed] [Google Scholar]
- 14.Raeven P, Feichtinger GA, Weixelbaumer KM, Atzenhofer S, Redl H, Van Griensven M, Bahrami S, and Osuchowski MF. 2012. Compartment-specific expression of plasminogen activator inhibitor-1 correlates with severity/outcome of murine polymicrobial sepsis. Thromb Res 129: e238–245. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Chen H, Cai J, Zou L, Yan D, Xu G, Li D, and Chao W. 2015. Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem 290: 26688–26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou L, Feng Y, Xu G, Jian W, and Chao W. 2016. Splenic RNA and MicroRNA Mimics Promote Complement Factor B Production and Alternative Pathway Activation via Innate Immune Signaling. Journal of immunology 196: 2788–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Zou L, Yan D, Chen H, Xu G, Jian W, Cui P, and Chao W. 2017. Extracellular MicroRNAs Induce Potent Innate Immune Responses via TLR7/MyD88-Dependent Mechanisms. J Immunol 199: 2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, and Chao W. 2018. Circulating Plasma Extracellular Vesicles from Septic Mice Induce Inflammation via MicroRNA- and TLR7-Dependent Mechanisms. J Immunol 201: 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, Kulkarni H, Wilhelmsen K, Warren S, and Hellman J. 2011. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol 186: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Kebir D, Damlaj A, Makhezer N, and Filep JG. 2015. Toll-Like Receptor 9 Signaling Regulates Tissue Factor and Tissue Factor Pathway Inhibitor Expression in Human Endothelial Cells and Coagulation in Mice. Critical Care Medicine 43: e179–e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivula M, Pettila V, Niemi TT, Varpula M, and Kuitunen AH. 2009. Thromboelastometry in patients with severe sepsis and disseminated intravascular coagulation. Blood Coagul Fibrinolysis 20: 419–426. [DOI] [PubMed] [Google Scholar]
- 22.Saraiva IE, Miranda PK, Freitas JN, Oliveira CR, Ataide TL, Nobre V, and Andrade MV. 2016. Thromboelastometric evaluation of sepsis associated coagulopathy: A cohort study. Thromb Res 147: 124–125. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, and Chao W. 2011. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286: 31308–31319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, and Betrosian A. 2008. Liver histology in ICU patients dying from sepsis: a clinico-pathological study. World journal of gastroenterology 14: 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spor A, Koren O, and Ley R. 2011. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279–290. [DOI] [PubMed] [Google Scholar]
- 26.Loh G, Brodziak F, and Blaut M. 2008. The Toll-like receptors TLR2 and TLR4 do not affect the intestinal microbiota composition in mice. Environ Microbiol 10: 709–715. [DOI] [PubMed] [Google Scholar]
- 27.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, and Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proceedings of the National Academy of Sciences of the United States of America 101: 5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharbert G, Kalb ML, Essmeister R, and Kozek-Langenecker SA. 2010. Mild and moderate hypothermia increases platelet aggregation induced by various agonists: a whole blood in vitro study. Platelets 21: 44–48. [DOI] [PubMed] [Google Scholar]
- 29.Hewlett L, Zupancic G, Mashanov G, Knipe L, Ogden D, Hannah MJ, and Carter T. 2011. Temperature-dependence of Weibel-Palade body exocytosis and cell surface dispersal of von Willebrand factor and its propolypeptide. PLoS One 6: e27314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, and Hartwig JH. 2003. The clearance mechanism of chilled blood platelets. Cell 112: 87–97. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder V, and Kohler HP. 2010. Thrombelastographic studies on factor XIII. Thromb Haemost 104: 1277–1279. [DOI] [PubMed] [Google Scholar]
- 32.Trelinski J, Pachniewska K, Matczak J, Robak M, and Chojnowski K. 2016. Assessment of Selected ROTEM Parameters, Kinetics of Fibrinogen Polymerization and Plasmin Amidolytic Activity in Patients with Congenital Fibrinogen Defects. Adv Clin Exp Med 25: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 33.Ketter PM, Guentzel MN, Schaffer B, Herzig M, Wu X, Montgomery RK, Parida BK, Fedyk CG, Yu JJ, Jorgensen J, Chambers JP, Cap AP, and Arulanandam BP. 2014. Severe Acinetobacter baumannii sepsis is associated with elevation of pentraxin 3. Infect Immun 82: 3910–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Hu D, He C, Wang T, Liu X, Ma L, Lin Z, and Chen Z. 2013. Novel biomarkers for early prediction of sepsis-induced disseminated intravascular coagulation in a mouse cecal ligation and puncture model. J Inflamm (Lond) 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel KN, Soubra SH, Lam FW, Rodriguez MA, and Rumbaut RE. 2010. Polymicrobial sepsis and endotoxemia promote microvascular thrombosis via distinct mechanisms. J Thromb Haemost 8: 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rex S, Beaulieu LM, Perlman DH, Vitseva O, Blair PS, McComb ME, Costello CE, and Freedman JE. 2009. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. Thrombosis and haemostasis 102: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, and Freedman JE. 2014. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood 124: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daudel F, Kessler U, Folly H, Lienert JS, Takala J, and Jakob SM. 2009. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Critical Care 13: R42–R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jian W, Gu L, Williams B, Feng Y, Chao W, and Zou L. 2019. Toll-like receptor 7 contributes to inflammation, organ injury, and mortality in murine sepsis. Anesthesiology In Press. [DOI] [PMC free article] [PubMed]
- 40.Recknagel P, Gonnert FA, Westermann M, Lambeck S, Lupp A, Rudiger A, Dyson A, Carré JE, Kortgen A, Krafft C, Popp J, Sponholz C, Fuhrmann V, Hilger I, Claus RA, Riedemann NC, Wetzker R, Singer M, Trauner M, and Bauer M. 2012. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS medicine 9: e1001338–e1001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.