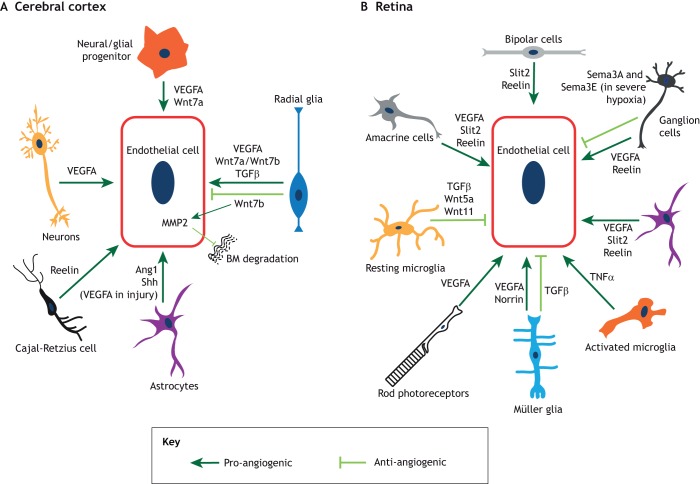
Fig. 4.
The main neuronal and glial-derived signals that influence angiogenesis and barriergenesis. Schematic representation of the signaling interactions between endothelial cells and other cell types in the cerebral cortex (A) and the retina (B). (A) In the cerebral cortex, radial glia, neuroglial progenitors and neurons secrete VEGFA. Radial glia and neuroglial progenitors also secrete several Wnt ligands; radial glia also secrete TGFβ. Astrocytes produce Ang1 and sonic hedgehog (Shh), and these same cells in the adult cortex secrete VEGFA during injury. Finally, Cajal-Retzius cells express reelin. Pro-angiogenic processes are depicted in dark green, whereas anti-angiogenic processes are depicted in light green. (B) In the retina, neuronal cell types and Müller glia secrete VEGFA, which facilitates vascular growth in distinct layers. Ganglion cells, amacrine cells and bipolar cells secrete reelin, whereas amacrine cells and bipolar cells produce Slit2, both of which promote angiogenesis. Depending on tissue physiology (such as severe ischemia), ganglion cells also secrete semaphorin 3A and semaphorin 3E (Sema3A and Sema3E), which inhibit retinal vascular growth. Müller glia secrete norrin, which promotes retinal angiogenesis and BRB formation. In contrast, Müller glia-derived TGFβ inhibits endothelial cell proliferation and vascular branching. TGFβ1 derived from resting microglia inhibits endothelial cell proliferation, while Wnt5a and Wnt11 produced by these cells negatively regulate vascular branching in the deep plexus. In contrast, activated microglia induce endothelial cell proliferation, likely by secreting tumor necrosis factor α (TNFα). Pro-angiogenic factors are depicted in dark green, whereas anti-angiogenic factors are depicted in light green.