Fig. 2. Cu-catalysed benzylic C–H functionalization with NFSI as the oxidant.
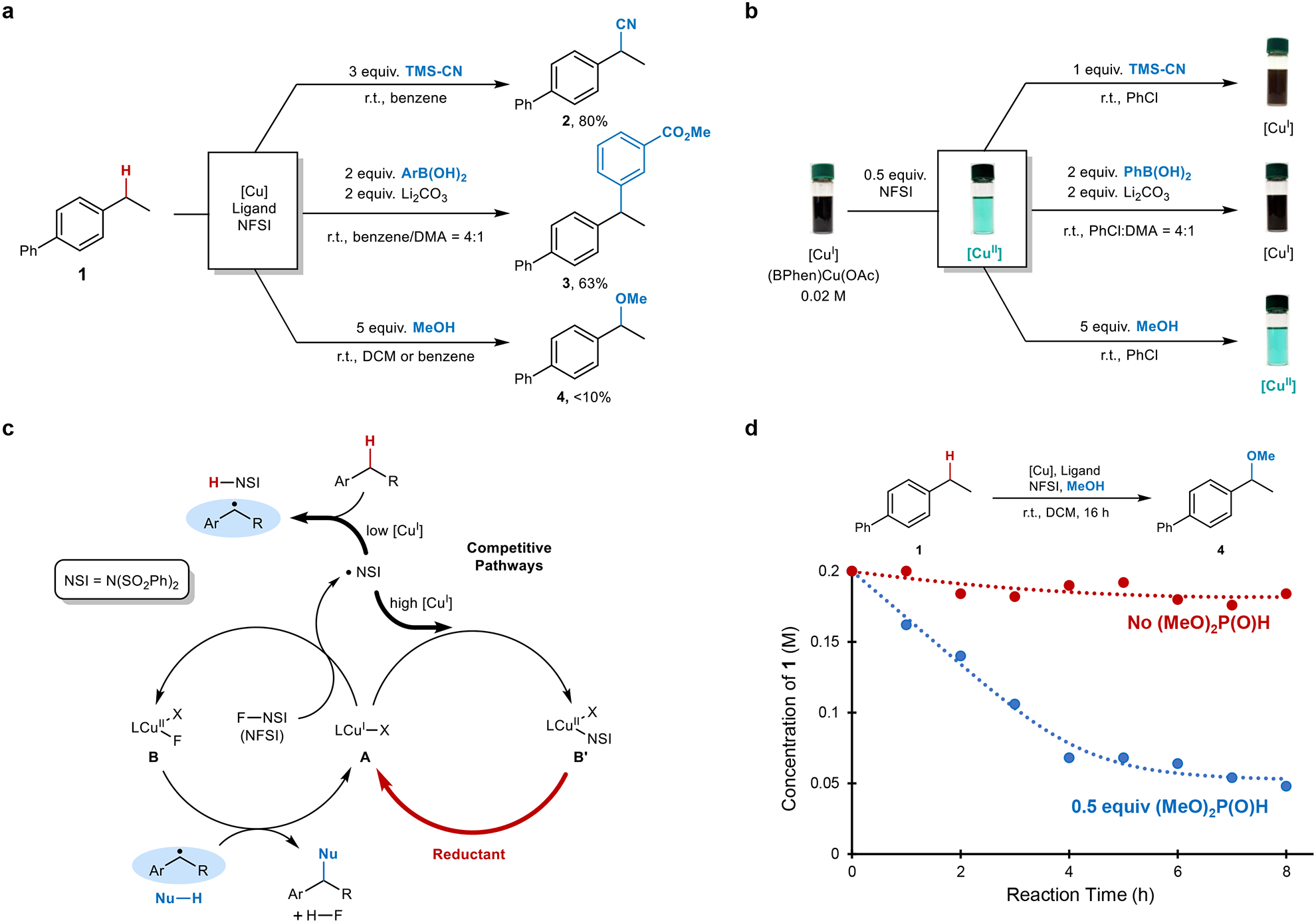
a, Cu-catalysed benzylic C–H functionalization reactions14,34. b, Changes in the Cu redox state between +1 (brown) and +2 (blue-green) upon addition of NFSI to a solution of the CuI catalyst precursor, followed by addition of cross-coupling partners. c, Modified radical relay mechanism (cf. Fig. 1c) to account for quenching of the •NSI by CuI and regeneration of CuI by a reducing substrate or additive. d, Reaction time course for benzylic etherification conducted in the absence (red) and presence of 0.5 equiv of dimethylphosphite (blue). Reaction conditions: 4-ethylbiphenyl (0.2 mmol), NFSI (0.4 mmol), MeOH (1.0 mmol), CuCl (0.02 mmol), 2,2’-bioxazoline (0.02 mmol), DCM (1 mL), room temperature.
