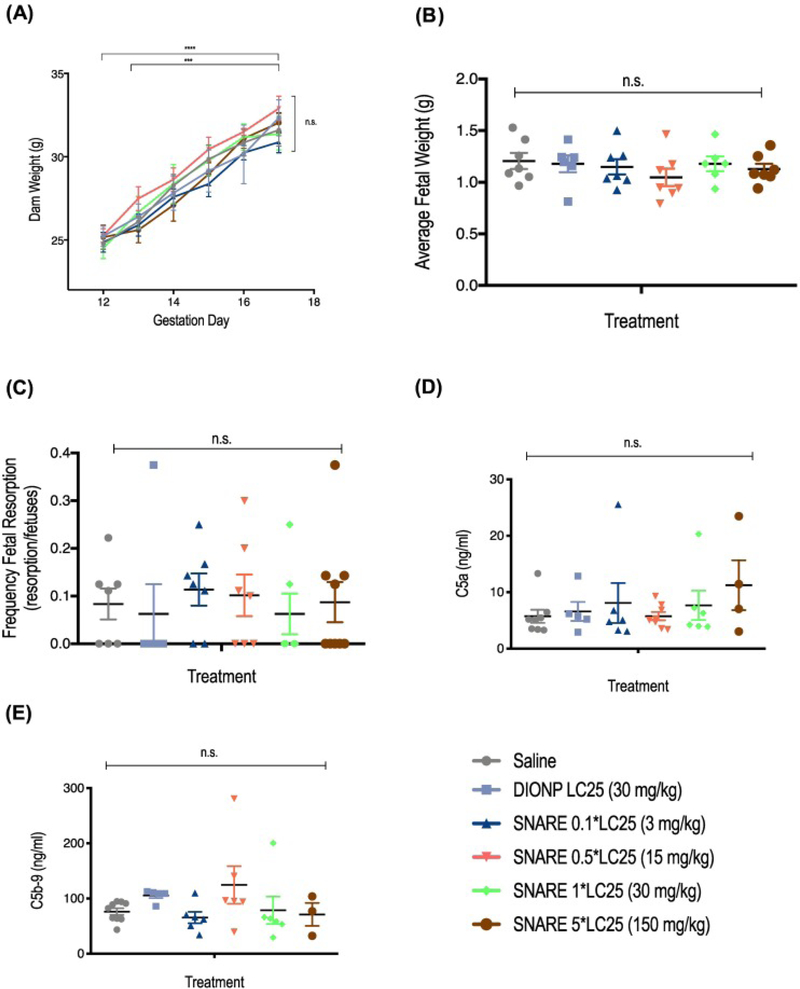
Figure 7.
SNARE maximum tolerated dose (MTD) and assessment of complement activation in pregnant dams. (A) Administration of SNAREs did not affect prenatal weight gain. Mice were weighted post-injection from GD 12 to GD 17, when mice were sacrificed for post-mortem analysis. (B) Average fetal weight (AFW), determined by dividing the total weight of the uteri by the number of non-resorbed fetuses, is shown. (C) Frequency of fetal resorption calculated as number of resorption/number of fetuses of treated compared to saline-treated mice. (D) C5a complement factor concentration in serum of mice treated with SNAREs showed no significant differences to the saline control. (E) C5b-9 complement factor concentration in serum of SNARE-treated mice also demonstrated no significant differences compared to saline control.