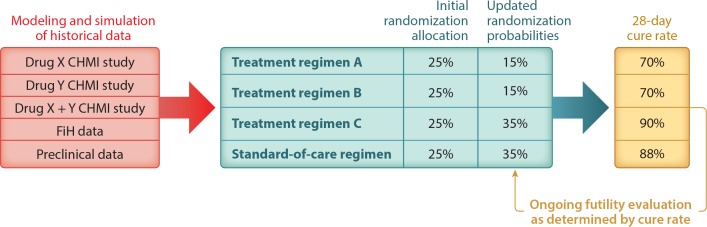
Figure 4.
Modeling and simulation using dose-response data generated from CHMI studies guides the study design and selection of treatment regimens. The platform trial design allows for interim analysis of outcomes and updates randomization probabilities based on response to treatments. This figure shows three treatment regimens for simplicity, but there may be many more early on, as treatment regimens may represent different doses of the drugs in combination. As the quality of the predictions improves, the number of treatment regimens for various doses of Drug X and Drug Y may change. Abbreviations: CHMI, controlled human malaria infection; FiH, first-in-human.