Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated disease (COVID-19), recently reported in Wuhan, is causing an unprecedented pandemia. Patients typically present with fever, cough and dyspnea. Advanced age, cardiovascular disease (CVD), diabetes (DM) and lung disease (LD) have been related with worse clinical outcome [1].
Limited information is available about the prognosis of this infection in immunosuppressed patients [2]. Here, we report a case of COVID-19 presenting in a patient diagnosed with advanced small cell lung cancer (SCLC) with a grade IV neutropenia.
On 20 of March, a 58 year-old Caucasian male, with a previous history of smoking and no CVD had been diagnosed of advanced SCLC in June 2019. He had an ECOG 2 performance status and was receiving Topotecan as second line therapy. During the COVID-19 outbreak, he was admitted with a two days history of fever (38.5−39 °C), cough, progressive asthenia, chest pain and expectoration. His oxygen saturation (O2 Sat) was 92 % on room air and respiratory rate was 35 breaths per minute. Lung auscultation showed a bilateral reduction of vesicular murmur in left lung and mild crepitants.
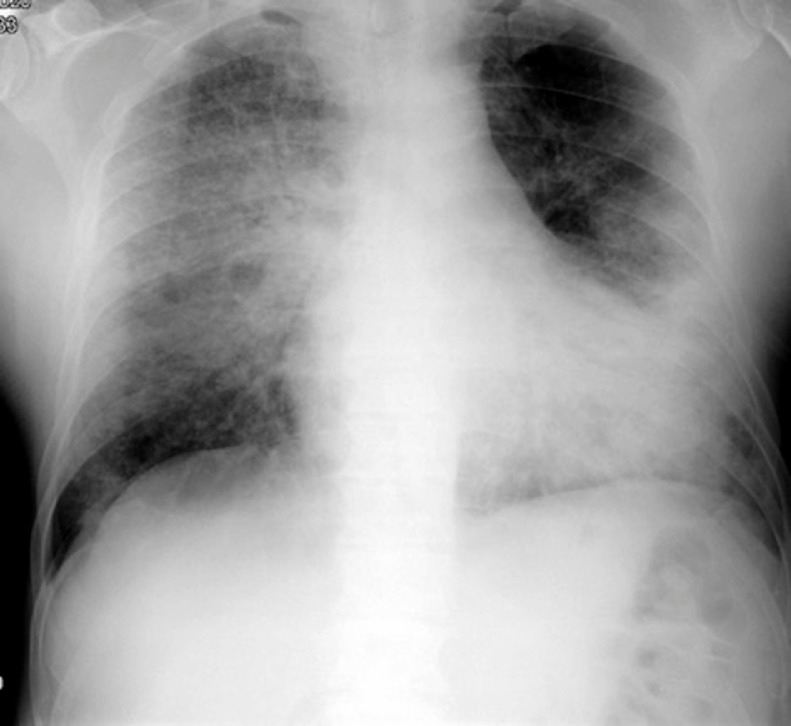
Most relevant laboratory findings were neutrophils 0.1 10E3/μL (LLN 1.5); lymphopenia 0.6 10E3/μL (LLN 1.1), platelets 44 10E3/μL (LLN 140.0) and high D-dimer value (2370 ng/mL). A chest X–Ray showed lower left lobe atelectasis and diffuse bilateral infiltrates (Fig. 1 ). Oropharyngeal swab PCR was positive for SARS-CoV-2. He received oxygen (FiO2 8 L/min), hydroxychloroquine and meropenem for one week to cover a possible bacterial coinfection, a high risk possibility according to very low neutrophils levels. He also received granulocyte stimulating growth factor (G-CSF) for 5 days. Due to cachexia-anorexia, daily dexamethasone 1 mg was kept. He was kept on 1 mg daily dexamethasone, that had been prescribed for anorexia-cachexia one month prior to admission.
Fig. 1.
The initial chest X–Ray, showing lower left lobe atelectasis and diffuse bilateral infiltrates, typical radiological findings of COVID19.
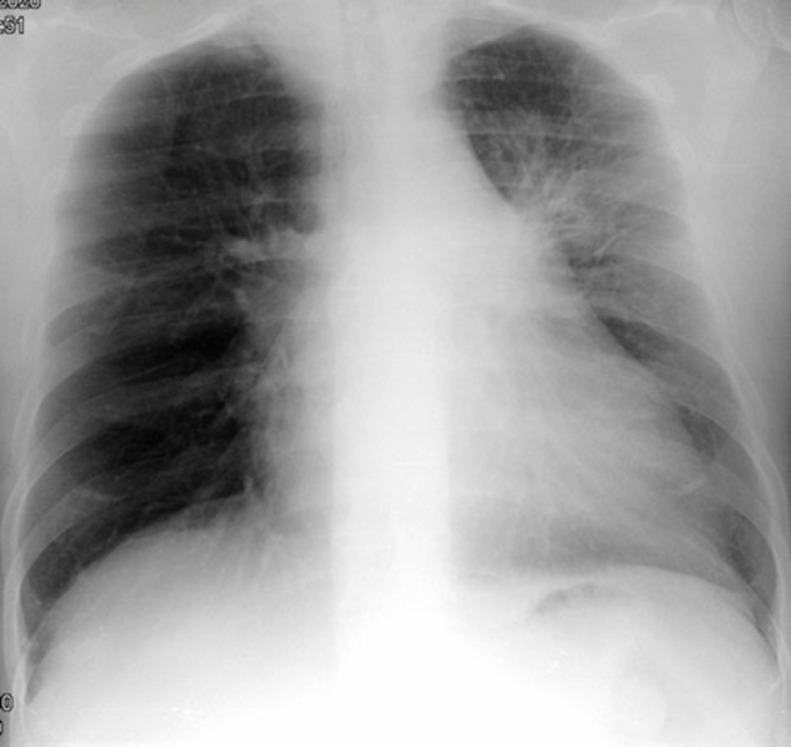
His clinical condition was improving gradually: lower Fi02 to maintain 95 % oxygen saturation; no fever since day 3; less cough, no chest pain and mild expectoration. At discharge on day 15, she had resolution of the respiratory symptoms, normal blood counts, including lymphopenia (Table 1 ), d-dimer value 390 and chest x-Ray showed a resolution of the initial diffuse bilateral infiltrates maintaining the pre-existing lower left lobe of the atelectasis (Fig. 2 ).
Table 1.
Evolution of the most relevant laboratory parameters in this patient in the hospital.
| 03/21 | 03/25 | 03/29 | 03/29 | 04/02 | |
|---|---|---|---|---|---|
| Leucocytes X 10E3/μL | 0.64 | 0.96 | 1.19 | 11.3 | 15.0 |
| Neutrophils X 10E3/μL | 0.1 | 0.1 | 0.3 | 8.0 | 8.1 |
| Lymphocites X 10E3/μL | 0.5 | 0.7 | 0.7 | 1.4 | 2.6 |
| Platelets X 10E3/μL | 27 | 9 | 26 | 30 | 67 |
| Haemoglobin g/dl | 11.7 | 10.1 | 9.9 | 9.5 | 9.5 |
| D-dimer ng/mL | 510 | 596 | 660 | 1030 | 1160 |
| LDH U/L | 156 | 169 | 200 | 258 | 201 |
| C.Protein Reactive mg/L | 247 | 91 | 77.3 | 54.6 | 50.3 |
Fig. 2.
The last chest X–Ray, showing a clear improvement of the bilateral infiltrates.
The United States Centers for Disease Control (CDC) agency includes IMS as a risk factor for severe illness. Liang et al. reported that cancer could be an aggravating factor based on 18 COVID-19 patients with cancer histories among a Chinese cohort of 1590 patients. In this small sub-analysis, they did not segregate by cancer type, stage, etc., and they observed that non-small cell lung cancer patients seemed to have a higher incidence of COVID-19, especially those with >60 years of age (4.3 % versus 1.8 % in those aged ≤60 years (1). Nevertheless, very limited data have been published about the potential risk for COVID-19 severe disease in cancer patients [2].
Some authors suggest that cancer induces an overexpression of immunosuppressive cytokines, dysfunctional leukocyte population and an abnormal dendritic cell maturation implying a lower risk for hyper inflammatory response (HIR) and SARS [3]. Therefore, all these events, could explain a lower risk for (HIR) associated to SARS-cov-2 infection after the initial virus clearance [4].
Consequently, in cancer patients could happened two opposite effects in SARS-cov2 infection setting: one effect could be a major vulnerability during the viral phase because of the cellular immunosuppression (IMS) and a second effect related with a certain protection for the HIR because of humoral as well as cellular IMS [5]. Frequent comorbidities in cancer like advanced age, CVD, DM, smoking status, LD may also modulate the risk.
In this patient, the young age with no CVD, and the presence of severe IMS and steroid treatment, could be hypothesized to explain the favorable prognosis. A recent case report published by Bomoni et al., described an advanced lung cancer patient treated with Nivolumab (antiPD-1) who presented a rapidly respiratory deterioration because COVID-19 disease. The lack of steroids administration could have influenced this course [6].
In our patient, neutropenia could have had a protective role. G-CSF receptor targeting in animal models of influenza pneumonia has been reported to reduce neutrophil trafficking and pulmonary edema without affecting viral clearance [7]. In addition, GM-CSF seems to play an important role in infection control by stimulating granulopoiesis and by improving microbicide functions of monocytes and granulocytes [8]. Also, GM-CSF targets Toll Like Receptors (TLR) that are crucial viral sensors and key players in the induction of inflammation. In this sense, targeting proinflammatory TLR signaling with GM-CSF has been suggested to for diseases where excessive inflammation leads to harmful outcome [9]. Following with the role of hematopoiesis stimulating factors in this disease, Hadadi et al. described a “miraculous” effect of erythropoietin (RhEPO) in a patient with SARS-Cov 2 infection, suggesting that RhEPO could modulate cytokine and leukocyte release [10].
Herein, we present an advanced cancer patient severely immunosuppressed and a favorable COVID-19 evolution. Immunosuppression provides a “calm cytokine scenario” that could shelter from COVID-19 induced “storm cytokine” HIR, thus protecting from severe disease. Furthermore, this report should draw attention to clinicians about how these patients could have a different prognosis. More epidemiological data are necessary to define the outcome of cancer patients with COVID-19 disease.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Competing Interest
We declare no competing interests.
Acknowledgements
To Eduardo Gutiérrez Gutiérrez for his contribution in English review and manuscript redaction.
References
- 1.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;11 doi: 10.1001/jama.2020.3786. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H., Li H., He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J.Y., Duan X.F., Wang L.P., Xu Y.J., Huang L., Zhang T.F., Liu J.Y., Li F., Zhang Z., Yue D.L., Wang F., Zhang B., Zhang Y. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J. Immunol. Res. 2014;28:286170. doi: 10.1155/2014/286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo P., Vieceli Dalla Sega F., Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res. Cardiol. 2020;115:31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica A., Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. 2017;85:117–125. doi: 10.1016/j.jaut.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Bonomi L., Ghilardi L., Arnoldi E., Tondini C.A., Bettini A.C. A rapid fatal evolution of Coronavirus Disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J. Thorac. Oncol. 2020;31 doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Aloe C., Wilson N., Bozinovski S. G-CSFR antagonism reduces neutrophilic inflammation during pneumococcal and influenza respiratory infections without compromising clearance. Sci. Rep. 2019;27:17732. doi: 10.1038/s41598-019-54053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleetwood A.J., Cook A.D., Hamilton J.A. Functions of granulocyte-macrophage colony-stimulating factor. Crit. Rev. Immunol. 2005;25:405–428. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi K., Wisgrill L., Wessely I., Diesner S.C., Schüller S., Dürr C., Heinle A., SacheT A., Pollak A., Förster-Waldl E., Spittler A. GM-CSF down-regulates TLR expression via the transcription factor PU.1 in human monocytes. PLoS One. 2016;11(11):e0162667. doi: 10.1371/journal.pone.0162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadadi A., Mortezazadeh M., Kolahdouzan K., Alavian G. Does recombinant human Erythropoietin administration in critically ill COVID-19 patients have miraculous herapeutic effects? J. Med. Virol. 2020;8 doi: 10.1002/jmv.25839. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]