Abstract
Background:
In previous studies, exposures to heavy metals such as Pb and Cd have been associated with adverse birth outcomes; however, knowledge on effects at low levels of exposure and of other elements remain limited.
Method:
We examined individual and mixture effects of metals and metalloids on birth outcomes among 812 pregnant women in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort. We measured 16 essential and non-essential metal(loid)s in maternal blood collected at 16–20 and 24–28 weeks gestation. We used linear and logistic regression to independently examine associations between geometric mean (GM) concentrations of each metal across visits and gestational age, birthweight z-scores, preterm birth, small for gestational age (SGA), and large for gestational age (LGA). We evaluated effect modification with infant sex*metal interaction terms. To identify critical windows of susceptibility, birth outcomes were regressed on visit-specific metal concentrations. Furthermore, average metal concentrations were divided into tertiles to examine the potential for non-linear relationships. We used elastic net (ENET) regularization to construct Environmental Risk Score (ERS) as a metal risk score and Bayesian Kernel Machine Regression (BKMR) to identify individual metals most critical to each outcome, accounting for correlated exposures.
Results:
In adjusted models, an interquartile range (IQR) increase in GM lead (Pb) was associated with 1.63 higher odds of preterm birth (95%CI=1.17, 2.28) and 2 days shorter gestational age (95% CI=−3.1, −0.5). Manganese (Mn) and zinc (Zn) were also associated with higher odds of preterm birth and shorter gestational age; the associations were strongest among the highest tertile for Mn and among females for Zn. Mercury (Hg) was associated with higher risk of preterm birth at the later window of pregnancy. Ni measured later in pregnancy was associated with lower odds of SGA. ENET and BKMR models selected similar metals as “important” predictors of birth outcomes. The association between ERS and preterm birth was assessed and the third tertile of ERS was significantly associated with an elevated odds ratio of 2.13 (95% CI= 1.12, 5.49) for preterm birth compared to the first tertile.
Conclusion:
As the PROTECT cohort has lower Pb concentrations (GM=0.33 μg/dL) compared to the mainland US, our findings suggest that low-level prenatal lead exposure, as well as elevated Mn and Zn exposure, may adversely affect birth outcomes. Improved understanding on environmental factors contributing to preterm birth, together with sustainable technologies to remove contamination, will have a direct impact in Puerto Rico and elsewhere.
Keywords: Metals, Birth outcomes, Biomarkers, Mixtures, Puerto Rico
INTRODUCTION
Preterm birth (<37 completed weeks of gestation) is a significant public health concern as it is the leading cause of infant mortality (Blencowe et al. 2013; Liu et al. 2012; Luu et al. 2016; Marlow et al. 2005). Other important adverse birth outcomes including low birthweight (<2500g) and being small for gestational age (SGA), which may result directly from preterm labor and/or growth restriction due to detrimental factors occurring during pregnancy, also contribute substantially to childhood and adult morbidity (Barker et al. 1989; Wigle et al. 2007; Yajnik et al. 1995).
Puerto Rico has one of the highest incidences of adverse birth outcomes among all US jurisdictions. In 2016, there were 3,248 preterm births in Puerto Rico, representing 11.5% of live births, compared to the national US average of 9.8% (Center for Disease Control and Prevention 2018). In addition, Puerto Rico has higher rates of childhood obesity and asthma (Garza et al. 2011; Otero-Gonzalez and Garcia-Fragoso 2008; Rivera-Soto et al. 2010) as well as obesity, metabolic syndrome, and diabetes in adults (Centers for Disease Control and Prevention 2012; Perez et al. 2008) compared to the rest of the U.S., all of which have been associated with higher rates of preterm birth and/or low birthweight. Moreover, traditional risk factors do not explain this high rate of adverse birth outcomes and associated consequences in Puerto Rico. Even though there is growing epidemiological (Amira M Aker et al. 2019; Ferguson et al. 2013; Ferguson et al. 2014; Murphy 2007) and toxicological (Berkowitz et al. 1996; Macones et al. 2004; Tardiff et al. 2006; Windham and Fenster 2008) evidence that environmental factors may play a key role, these factors remain understudied and underappreciated. Therefore, it is important to understand the role of environmental chemicals in adverse health outcomes and to develop new methods for reducing harmful exposures in Puerto Rico and beyond.
Ubiquitous in the environment, metals and metalloids have been widely detected among the U.S. population (Centers for Disease Control and Prevention (CDC) 2019), including pregnant women and their fetuses because of the trans-placental metal transfer (Caserta et al. 2013; Chen et al. 2014; Punshon et al. 2016). While many human and animal studies have focused on elucidating the effects associated with heavy metals cadmium (Cd), mercury (Hg), arsenic (As), and lead (Pb), less attention has been given to the other metals. However, a growing body of evidence suggests that certain essential or trace metals, including copper (Cu) (Kim et al. 2018) and Nickel (Ni) (Chen et al. 2018), may be associated with an increased risk of preterm delivery. A few other studies also reported inverted U-shaped dose-response curves for the associations between birth weight and maternal metal exposures, including cobalt (Co) (Mikelson et al. 2018) and manganese (Mn) (Ashley-Martin et al. 2018; Zota et al. 2009). Therefore, there is a pressing need to study the effects of excessive exposure to essential trace elements on adverse pregnancy outcomes. In addition, most previous reports on the effects of metals on pregnancy are from studies usually involving high doses (e.g., studies on Pd before the elimination in paint and gas), not commonly encountered by pregnant women and fetus (Andrews et al. 1994; Ferguson et al. 2016). Due to the widespread exposure of humans and known toxicity of these metals, concern is growing that low-level exposure may also adversely affect birth outcomes and several birth cohorts have evaluated the health effects of low-level exposure to metals during pregnancy (Anderson et al. 2016; Ashley-Martin et al. 2018; Eum et al. 2014; Silver et al. 2016; Thomas et al. 2015; Wu et al. 2017).
As humans are continuously exposed to a mixture of environmental toxicants, there is a pressing need to study the relationship of exposures both individually and as mixtures (Centers for Disease Control and Prevention 2009). While most studies on metals have assessed metal exposures individually rather than in combination, a few have explored metal mixtures in relation to adverse birth outcomes (Govarts et al. 2016; Kim et al. 2018; Lee et al. 2019; Luo et al. 2017; Signes-Pastor et al. 2019). Compared to these earlier studies, our study has one of the largest numbers of toxic and trace metal analytes. Therefore, we investigated the effects of metal(loid)s on adverse birth outcomes both individually and as mixtures. Identifying modifiable environmental risk factors for adverse birth outcomes could have a positive public health impact if future exposures can be reduced through contaminant remediation or other exposure reduction strategies in an effort to reduce rates of preterm birth and other adverse health effects.
METHODS
2.1. Study Population
This study used data collected from 812 pregnant women participating in the ongoing prospective cohort project “the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT)” (Ashrap et al. 2018; Cantonwine et al. 2014; Meeker et al. 2013; Watkins et al. 2015). The PROTECT study launched in 2010 with funding from the NIEHS Superfund Research Program and conducted in Puerto Rico because of its high preterm birth rate and the extent of hazardous waste contamination on the island. PROTECT aims to explore environmental toxicants and other factors contributing to preterm birth risk and other adverse birth outcomes in Puerto Rico.
Study participants were recruited at approximately 14 ± 2 weeks of gestation at seven prenatal clinics and hospitals throughout Northern Puerto Rico and followed until birth. (Cantonwine et al. 2014; Meeker et al. 2013). Inclusion criteria for this study were: maternal age between 18 to 40 years; residence inside of the Northern Karst aquifer region; disuse of oral contraceptives within the three months prior to pregnancy; disuse of in vitro fertilization to become pregnant; and free of any major medical or obstetrical complications, including pre-existing diabetes. Each woman participated in a total of up to three study visits (18 ± 2 weeks, 22 ± 2 weeks, and 26 ± 2 weeks of gestation). Detailed information on medical and pregnancy history were collected at the initial visit. During an in-home visit (second visit), nurse-administered questionnaires were used to gather information on housing characteristics, employment status, and family situation. Blood samples were collected from women at the first and third visits. The present analysis reflects 812 women who delivered live singleton births in PROTECT and had metal biomarker measurements available.
The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan, Northeastern University, and the University of Georgia. The study was described in detail to all participants, and informed consent was obtained prior to study enrollment.
2.2. Measurement of Metals
Blood samples were collected in metal-free whole blood tubes and frozen at −80°C, and shipped on dry ice to NSF International (Ann Arbor, MI, USA) for analysis. Concentrations of 16 metals and metalloids: As, barium (Ba), beryllium (Be), Cd, Co, chromium (Cr), cesium (Cs), Cu, Hg, Mn, Ni, Pb, titanium (Ti), uranium (U), vanadium (V), and zinc (Zn) were measured in blood samples, using a Thermo Fisher (Waltham, MA, USA) ICAPRQ inductively coupled plasma mass spectrometry (ICPMS) and CETAC ASX-520 autosampler, as described previously (Kim et al. 2018). Standards of known purity and identity were used during the preparation of the calibration, quality control, and internal standards. The ICPMS was calibrated with a blank and a minimum of 4 standards for each element of interest. The calibration curve response versus concentration was evaluated for goodness of fit. All validated analyte correlation coefficients (R) were ≥ 0.995.
2.3. Gestational Age and Preterm Birth Calculation
All the birth outcome data were extracted from medical records. The American Congress of Gynecologists (ACOG) recommendations for best obstetrical estimate to calculate the gestational age for complete pregnancies (Committee on Obstetric Practice and Medicine; 2017) were used in our study to as previously described (A. M. Aker et al. 2019; Ferguson et al. 2019). Per common practice, preterm birth (premature labor) was defined as delivery < 37 completed weeks of gestation. Based on the presentation of preterm delivery, preterm birth was further classified as spontaneous preterm birth (presentation of premature rupture of the membranes, spontaneous preterm labor, or both) and non-spontaneous preterm birth (preterm births with preeclampsia or with both artificial membrane rupture and induced labor). We included overall and spontaneous preterm birth as two of the birth outcomes in our analysis.
2.4. Birthweight calculations
Birthweight z-scores (defined as the number of standard deviations by which a birthweight is above or below the mean) are commonly used to compare individual birthweights with the cohort (Griffin 2014; Kramer et al. 2001). Gestational age- and sex- specific birthweight z-score were constructed according to the INTERGROWTH-21st standards (Villar et al. 2014). Small for gestational age (SGA) births were defined as below the 10th percentile of birthweight z-scores. Large for gestational age (LGA) births were defined as above the 90th percentile of birthweight z-scores.
2.4. Statistical Methods
Biomarker concentrations below the limit of detection (LOD) were replaced by LOD/√2 (LOD). For statistical analysis, we included metal(loid)s with at least 70% of samples having concentrations above the LOD as continuous variables, and metal(loid)s with less than 70% of samples above the LOD (As and Cd) as binary variables (above vs below LOD). Metals with low detection rate (<30%) were excluded from the analyses. Descriptive statistics were calculated for all exposure and outcome variables. Log-transformed t-test was performed to compare the maternal metal concentrations between preterm and term births.
2.4.1. Single-Pollutant Models
Logistic regression models were used to examine the associations between metal exposure and binary adverse birth outcomes, including preterm birth (overall and spontaneous preterm birth), SGA, and LGA. As SGA and LGA may have similar complications, SGA models excluded LGA births, and LGA models excluded SGA births. Multiple linear regression was used to model metal exposures with continuous outcomes, gestational age and birthweight z-score. All outcomes are regressed on the geometric averages of participant concentrations across the two visits (when missing concentrations at one visit, the “average” concentration was equal to the single available concentration), with separate models for each exposure biomarker. Metal concentrations were natural log-transformed as they had right skewed distributions.
The crude models only included the geometric average blood metal concentration. The final set of covariates were selected in a stepwise procedure if they altered the beta coefficient of metal exposure by 10% or more. The covariates considered were maternal age, insurance type, maternal education level (an indicator of socioeconomic status), marital status, employment status, gravidity, pre-pregnancy BMI, smoking, exposure to second-hand smoking and alcohol consumption. The final models were controlled for maternal age, maternal education level, pre-pregnancy BMI, and exposure to second-hand smoking.
To assess potential windows of vulnerability in pregnancy, we fit separate multiple linear regression models for each visit using visit-specific metal concentrations. In another analysis, we divided average metal concentrations into tertiles to examine the potential for non-linear relationships. For non-essential metals, effect estimates were calculated for each of the top two tertiles in comparison to the lowest tertiles of exposure. For essential metals, effect estimates were calculated for the highest and lowest tertiles in comparison to the middle tertile of exposure (Kim et al. 2018).
Finally, to understand whether the effect estimates for metals on birth outcomes differed according to infant sex, all previously mentioned single-pollutant models were refitted with the addition of an interaction term between infant sex and metal concentration, and the interaction term coefficient was tested for significance.
The results were presented as change in days of gestational age and birthweight z-score (95% confidence intervals), and odds ratio of preterm birth, SGA and LGA (95% confidence intervals), per interquartile range (IQR) increase in metal concentrations. The alpha level was set at 0.05. We also considered significance after adjusting for multiple testing using the Benjamini-Hochberg method (Benjamini and Hochberg 1995). Since birth outcomes were correlated, we calculated q values (adjusted p values) treating each outcome as a family of tests (10 tests per outcome). A cutoff of 0.1 for q value was used to further interpret main results with greater confidence.
2.4.2. Mixture Analysis
In addition to analyzing each metal separately, we explored the effect of the metal mixture on birth outcomes with two approaches.
Elastic Net (ENET) and Metal Risk Score
In the first method, we constructed a metal risk score --Environmental Risk Score (ERS). An ERS is conceptualized as a weighted summary measure of the effects of multiple exposures where the weights are regression coefficients derived from a model of the association between chemical mixtures and the outcome of interest. We utilized elastic net (ENET) to identify the important metals that were driving the association with birth outcomes and to construct the ERS (Park et al. 2017). ENET is a regularized regression method that combines the penalties of the least absolute shrinkage and selection operator (LASSO) and ridge regression (Zou 2006). The objective function for a continuous outcome can be expressed as:
where i = 1, …, n indexes the subjects, is the vector of p covariates for the i-th subject, and yi is the continuous health outcome for the i-th subject. ENET utilizes two tuning parameters (λ, α). Intuitively, λ ∈ [0, ∞) controls the overall strength of the shrinkage while α ∈ [0,1] controls the tradeoff between automatic variable selection (L1 penalty) and stabilization of the solution path in the presence of collinear exposures (L2 penalty) (Zou and Zhang 2009). Therefore, ENET is generally considered a useful penalized regression approach for variable selection in the presence of highly collinear predictor variables (Park et al. 2017).
We fit ENET on all the metals (IQR standardized) in relation to each birth outcome of interest adjusted for the same covariates from the single-pollutant analysis (retained in the model, not subject to shrinkage). Tuning parameters were selected using 10-fold cross-validation. ERS was computed as a weighted sum of the selected non-zero predictor coefficients from each model. We further categorized ERS by tertiles and refit the regression models to examine the associations between categorical ERS and birth outcomes and compared results to those from individual tertile models.
Bayesian kernel machine regression (BKMR)
The second approach for conducting mixture analysis was Bayesian kernel machine regression (BKMR) (Bobb et al. 2015), which enabled us to evaluate the joint effect of multiple metals, interactions between metals, and potential non-linear relationships between metals and outcomes of interest (Bobb et al. 2018). Because exposures in our study are correlated, we implemented BKMR with hierarchical variable selection (10,000 iterations by a Markov Chain Monte Carlo (MCMC) algorithm). This approach requires grouping of exposures based on correlations between exposures (SI Figure S1) and similar potential mechanisms of action (e.g. toxic metals vs essential metals). Therefore, we grouped As, Cd, Hg, and Pb into group 1 (toxic metals), Co and Mn into group 2 (correlated essential metals), and Cs, Cu, Ni, and Zn (essential metals) into group 3. Posterior inclusion probabilities (PIP) were extracted from each BKMR model, which provides a measure of variable importance for each exposure group (groupPIP) and how each exposure in that group is driving that group-outcome association (condPIP). To determine the importance of each group/exposure for each study outcome a threshold of PIP>0.5 was used (Coker et al. 2018; Zhang et al. 2019).
Data were analyzed using R version 3.6.2 and SAS 9.4 (SAS Institute Inc., Cary, NC)
RESULTS
Demographic characteristics of 812 women in our analysis were described previously (A. M. Aker et al. 2019; Ashrap et al. 2018) and summarized in Table S1. The mean age of participants was 26.7 and nearly half of the women had a BMI less than 25kg/m2 prior to pregnancy. Approximately two-thirds of the women in our study had private insurance providers and were employed. 30% had reported graduating from college or higher. Nearly half of them had annual household incomes less than $30,000. 80% of the women never smoked while very few (6.8%) reported consumption of alcohol within the last few months. Mean gestational age was 38.8 (SD=2.1) weeks for 812 singleton births included in this analysis, among which 80 (10%) were preterm and 48 (6%) were spontaneous preterm; the rates of SGA and LGA were both 9%.
Descriptive statistics and Spearman correlations between different metals were previously reported elsewhere (Ashrap et al. 2020). Briefly, 1) all metals were detected in the majority of samples, with the exception of As (49% > LOD) and Cd (61% > LOD) and Ba, Be, Cr, Ti, U, and V, which were detected in very few samples (<30%) (SI Table S2); 2) mean concentrations of Cu, Mn, Pb, and Zn were higher for preterm birth cases compared to other births. 3) there were weak to moderate correlations between different blood metal concentrations (Mn and Co, r=0.36; As and Hg, r=0.32; Cd and Co, r=0.33, SI Figure S1); 4) the first visit had lower concentrations of Cd, Co, Cu, Mn, and Zn, and higher concentrations for Cs, when comparing to the third visit; and 5) Cu, Zn, Pb, Mn, and Hg presented good to excellent reliability in repeated blood samples with intraclass correlation coefficients (ICCs) ranging from 0.54–0.78.
3.1. Single-pollutant Metal Analyses
The full models included 731 women who had complete data on the four demographic covariates (age, maternal education, pre-pregnancy BMI, and second-hand smoking). Table 1 presents the associations between average metal concentrations and birth outcomes, while Figure 1 and SI Table S3 show the visit specific associations.
Table 1.
Change in gestational age, preterm birth, and SGA associated with average exposure biomarker concentration across two time points during pregnancy. Effect estimates presented as changes or odds ratio (OR) for IQR increase in exposure biomarker concentration. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
| Metals | Gestational age (N=731) | Preterm Birth (overall, N=731) | Preterm Birth (spontaneous, N=700) | SGA (N=637) | ||||
|---|---|---|---|---|---|---|---|---|
| Co | −1.1 (−2.3, 0.1) | 0.06 | 1.21 (0.93, 1.56) | 0.16 | 1.33 (0.96, 1.84) | 0.09 | 0.9 (0.68, 1.21) | 0.49 |
| Cs | −0.3 (−1.6, 11) | 0.69 | 1.19 (0.84, 1.68) | 0.33 | 1.1 (0.72, 1.69) | 0.66 | 0.82 (0.62, 1.08) | 0.15 |
| Cu | −0.4 (−1.2, 0.4) | 0.36 | 1.32 (0.98, 1.78) | 0.07 | 1.2 (0.82, 1.76) | 0.34 | 0.99 (0.83, 1.19) | 0.94 |
| Mn | −1.1 (−2.4, 0.3) | 0.12 | 1.32 (0.96, 1.8) | 0.08 | 1.45 (0.98, 2.15) | 0.07 | 0.85 (0.62, 1.16) | 0.30 |
| Ni | 0.8 (−0.3, 1.9) | 0.15 | 0.85 (0.65, 1.11) | 0.24 | 0.85 (0.6, 1.21) | 0.36 | 0.67 (0.49, 0.9) | 0.01* |
| Zn | −0.7 (−1.5, 0.2) | 0.11 | 1.83 (1.28, 2.6) | 0.001* | 1.53 (0.99, 2.38) | 0.06 | 1 (0.82, 1.21) | 1.00 |
| Asa | 1.8 (−0.4, 3.9) | 0.10 | 0.72 (0.44, 1.18) | 0.19 | 0.65 (0.34, 1.24) | 0.19 | 0.75 (0.45, 1.25) | 0.28 |
| Cda | −1.3 (−3.5, 0.9) | 0.26 | 1 (0.6, 1.65) | 0.99 | 1.24 (0.63, 2.43) | 0.53 | 0.76 (0.45, 1.27) | 0.29 |
| Hg | 0.8 (−0.6, 2.2) | 0.27 | 1.05 (0.75, 1.48) | 0.76 | 1.27 (0.82, 1.95) | 0.28 | 0.86 (0.62, 1.21) | 0.40 |
| Pb | −1.8 (−3.1, −0.5) | 0.009* | 1.63 (1.17, 2.28) | 0.004* | 1.53 (1, 2.35) | 0.05 | 0.91 (0.69, 1.2) | 0.51 |
As, Cd were compared between two categories of above LOD and below LOD
q value (false discovery rate) <0.1
Figure 1.
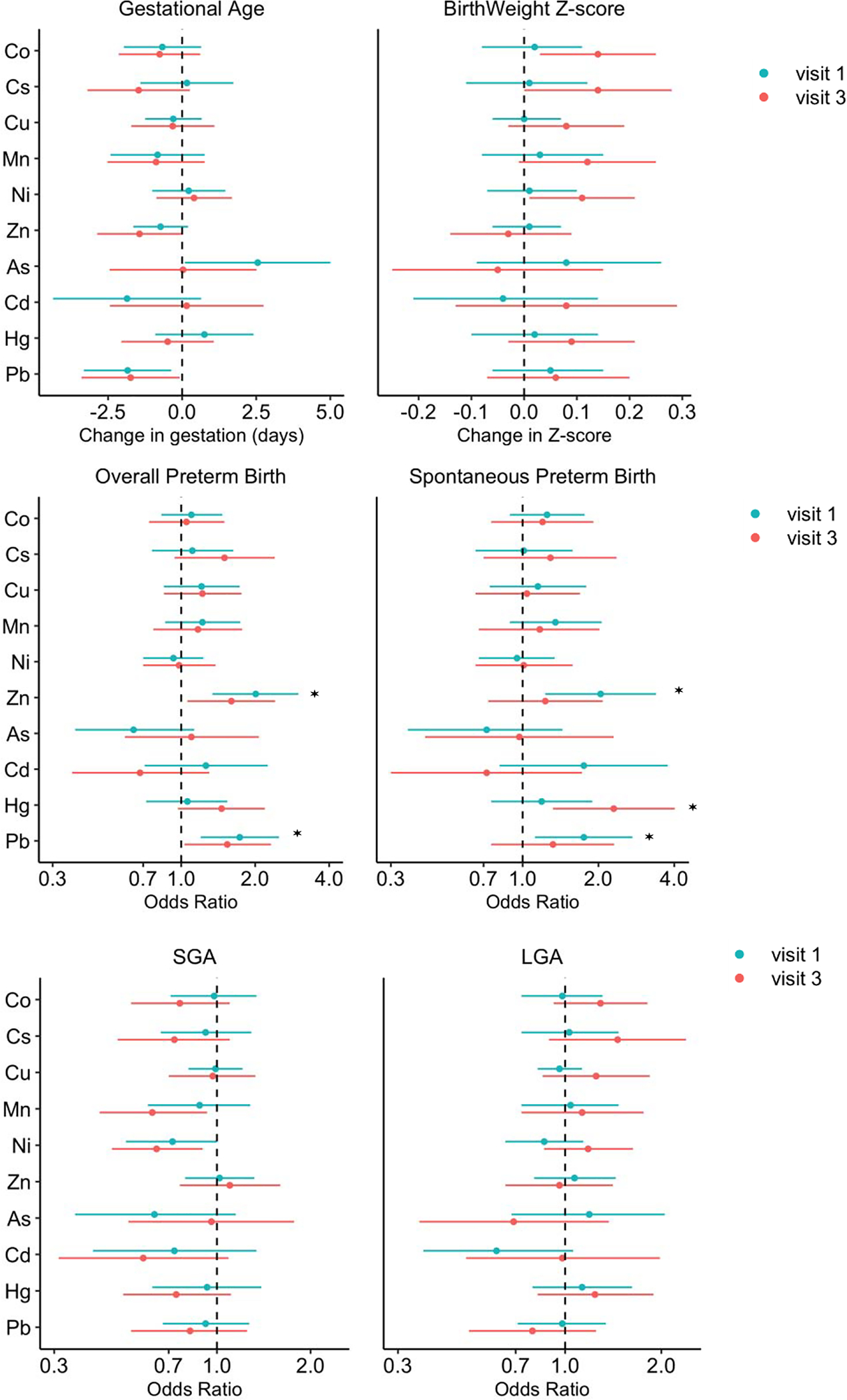
Change in birth outcomes associated with visit specific exposure biomarker concentration. Effect estimates presented as changes or odds ratio (OR) for IQR increase in exposure biomarker concentrationa. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
a As, Cd were compared between two categories of above LOD and below LOD
*q value (false discovery rate) <0.1
GA and birthweight
Average Pb concentration was strongly associated with gestational age, for which an IQR increase was associated with 2 days (95% CI=−3.1, −0.5; q value=0.09) shorter gestational age; the effect estimates did not differ by study visit. Average Zn was also suggestively associated with decreased gestational age (△/IQR= −0.7, 95% CI= −1.5, 0.2) and the association with third visit Zn remained significant, after stratification of the results by study visit (Figure 1 and SI Table S3).
No significant relationships were observed between birthweight z-score and average metal concentrations (data not shown). However, as shown in Figure 1, when stratified on study visit, third visit Co, Cs, Ni concentrations were significantly positively associated with birthweight z-score, with an increase of 0.14 (95% CI=0.03, 0.25), 0.14 (95% CI= 0.00, 0.28), and 0.11 (95% CI= 0.01, 0.21) in birthweight z-score per IQR increase in the metal concentrations, respectively (SI Table S3).
Preterm birth, SGA, LGA
In line with results from gestational age analysis, average Pb, Mn, and Zn concentrations were associated with elevated odds of preterm birth (both overall and spontaneous), with OR ranging from 1.32 to 1.83 per IQR increase in metal concentration (Table 1). In the stratified analysis, Pb and Zn were associated with increased odds of spontaneous preterm birth at only visit 1 (Pb: OR/IQR= 1.75, 95% CI: 1.12, 2.73; Zn: OR/IQR= 2.04, 95% CI= 1.23, 3.39). In a sensitivity analysis where metal concentrations were entered in models as tertiles, the change in gestational age and odds of having a preterm birth was only significant among the highest tertile for Pb and Mn (Figure 2 and SI Table S4).
Figure 2.
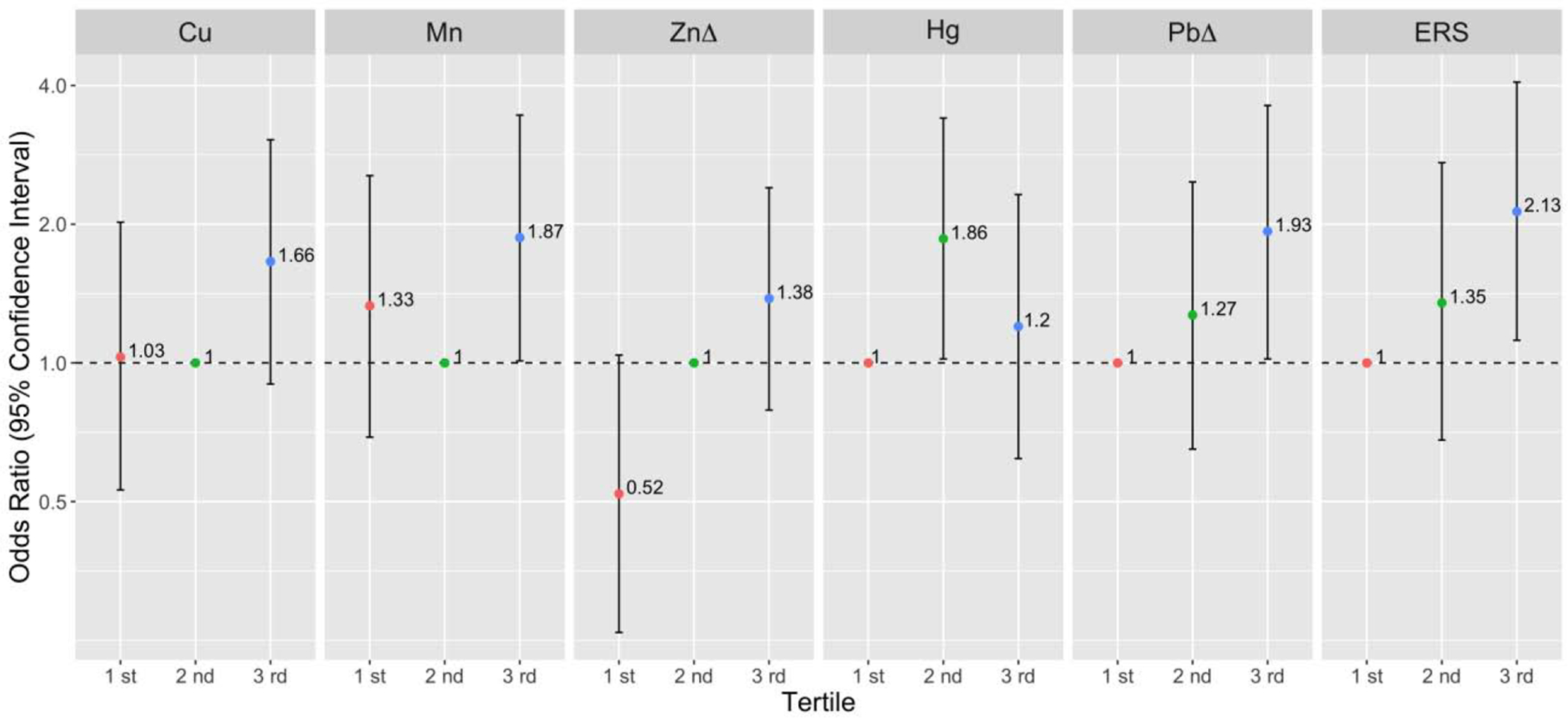
Preterm birth (overall) odds ratio (95% confidence interval) associated with tertiles of geometric average exposureab Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
a Referent levels were set at tertile 2 for essential metals (Cu, Mn, Zn).
b Referent levels were set at tertile 1 for non-essential metals (Hg, Pb), and environmental risk score (ERS). Δ Individual metals that were selected from elastic net models to compose ERS.
Though geometric mean average models for Hg did not find any association with birth outcomes, Hg was associated with 1.5- and 2.3-fold increased odds of overall and spontaneous preterm birth at visit 3 (overall: OR/IQR= 1.46, 95% CI= 0.97, 2.19; spontaneous: OR/IQR= 2.30, 95% CI= 1.32, 4.02), respectively (Figure 1); interestingly, this association appeared stronger when comparing women in lower two tertiles of exposure rather than higher two tertiles (Figure 2 and SI Table S4). For SGA, Ni concentration was associated with decreased OR, although only significant when comparing the highest tertile to the middle tertile (OR/IQR= 0. 33, 95% CI= 0.16, 0.66). Visit specific analysis also revealed that higher Mn concentration at the third visit was associated with decreased odds of SGA (OR/IQR= 0.62, 95% CI= 0.42, 0.93). No metal concentrations were associated with LGA in average and visit stratified models (SI Table S3).
After correcting for multiple testing, the associations of both average and first visit Pb and Zn with overall preterm birth, third visit Hg with spontaneous preterm birth, as well as the association of average Ni with SGA had q-values < 0.1 (Table 1, Figure 1), providing greater confidence in these associations.
3.2. Mixture analyses
Table S5 shows the variable selection results from ENET models. The estimated weights (regression coefficients) presented in Table S5 are from models where metal concentrations were log transformed and IQR standardized. Preterm birth (overall) models had more than one metal with non-zero weights; Pb (β = 0.057, OR=1.06) and Zn (β = 0.011, OR=1.01) were selected as important predictors and all other metals were shrunk to zero. Therefore, we constructed ERS using estimated weights for Pb and Zn and regressed preterm birth by this score. The OR for preterm birth comparing the highest vs. the lowest tertiles of ERS was 2.13 (95% CI= 1.12, 5.49, p=0.02) (Figure 2). In the BKMR hierarchical variable selection models for preterm birth, all three metal groups had posterior inclusion probabilities higher than 0.5 and the important metals selected from the groups included Zn (condPIP=0.83), Pb (condPIP=0.68), and Mn (condPIP=0.60). In a secondary analysis, we ran BKMR models regressing preterm birth while only including Mn, Zn, Pb to explore the potential non-linearity and interaction between the predictors. The single metal-response curves in Figure 3 show that 1) Pb and Zn had a positive linear relationship with preterm birth at higher levels (the confidence intervals at lower distributions are wide due to sparse data); 2) the overall trend for Mn was also positive and generally linear. Further, the associations between each metal and preterm birth did not differ by varying quantiles of the other two metals, indicating a lack of interaction between different metals (Figure 3B).
Figure 3.
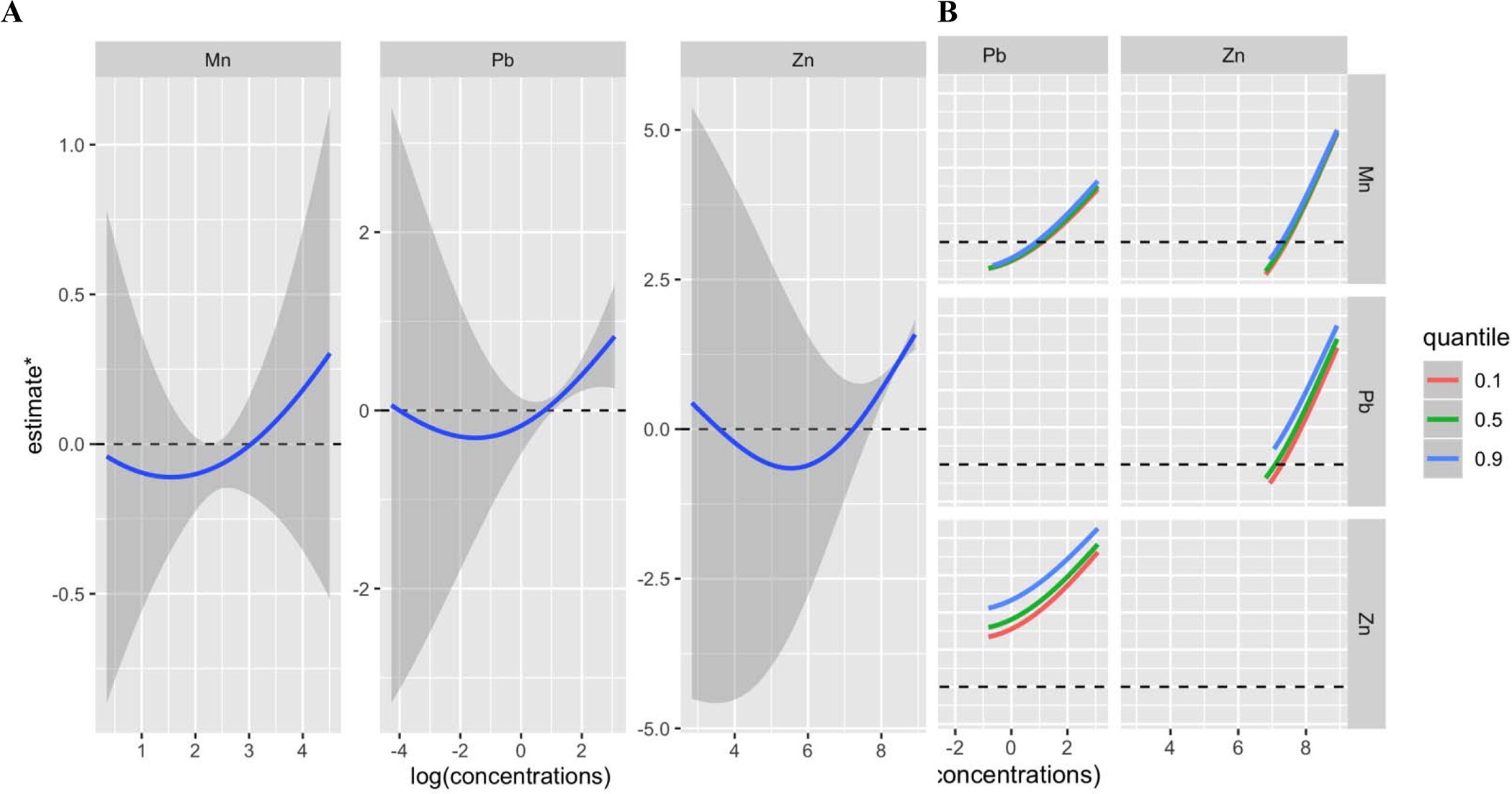
Univariate and bivariate predictor-response function for the effect of metal mixture (Mn, Pb, Zn) on preterm birth estimated by Bayesian Kernal Machine Regression (BKMR)a. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
a estimate* may be interpreted as a latent continuous marker of the binary outcome-overall preterm birth A Univariate exposure–response functions and 95%confidence for each metal with the other pollutants fixed at the median B Bivariate exposure–response functions for: Mn when Pb is fixed at either the 10th, 50th, or 90th percentile and Zn is fixed at the median (middle left panel); Mn when Zn is fixed at either the 10th, 50th, or 90th percentile and Pb is fixed at the median (bottom left panel); Pb when Mn is fixed at either the 10th, 50th, or 90th percentile and Zn is fixed at the median (top middle panel); Pb when Zn is fixed at either the 10th, 50th, or 90th percentile and Mn is fixed at the median (bottom middle panel); Zn when Mn is fixed at either the 10th, 50th, or 90th percentile and Pb is fixed at the median (top right panel); Zn when Pb is fixed at either the 10th, 50th, or 90th percentile and Mn is fixed at the median (middle right panel);
3.3. Sex interaction
When interactions between infant sex and metal concentrations were added to single-pollutant models, the interaction terms were not statistically significant, except for the associations between Zn and gestational age (p value=0.01), and Cu and LGA (p value=0.03). Stratified analysis by infant sex showed that the effect of Zn on gestational age was only significant among female infants (p value=0.006) but not male infants (p value=0.62); one IQR increase in Zn was associated with 3 days (95% CI= −5.2, −0.9) shorter gestational age among women who delivered female infants. Figure 4 shows the interaction effect of infant sex on the association between the average Zn concentration and gestational age. The impact of Cu on LGA also varied by infant sex, where odds of LGA were reduced among female infants (OR/IQR= 0.62, 95% CI= 0.39, 0.99, p value=0.04). However, differences in associations between metals and birth outcomes by sex were not observed when we conducted the mixture analyses stratified by infant sex. Only Pb was identified as the important predictor of preterm birth/gestational age from ENET and BKMR models, among both female and male infants.
Figure 4.
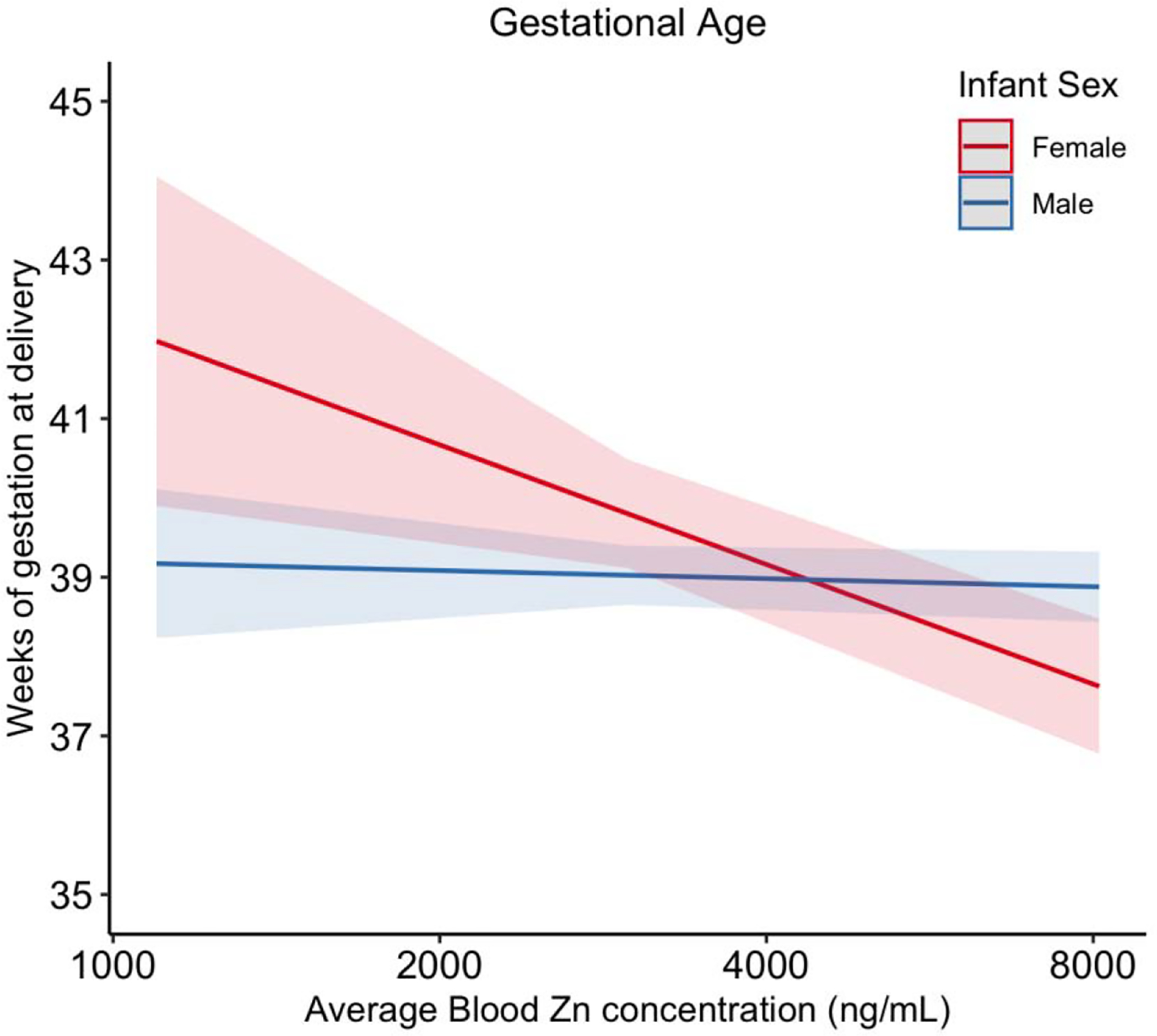
Interaction effect of infant sex on the association between the average Zn blood concentration and gestational age. Models were adjusted for maternal age, maternal education, pre-pregnancy BMI, and exposure to secondhand smoking.
The results from tertile analyses stratified by infant sex and study visit were similar to the main tertile analyses results we reported; sex-specific interactions were not observed.
DISCUSSION
In this study, we evaluated the individual effects of prenatal essential and non-essential metal(loid) exposure on adverse birth outcomes among a Puerto Rico population. Our analyses demonstrated that maternal blood concentrations of Pb, Zn, Mn, and Hg were associated with shorter gestational age and higher odds of preterm birth, while Ni was associated with higher birthweight and lower odds of SGA. Some associations were observed only when considering exposure at specific prenatal timepoints, which may reflect windows of exposure vulnerability. Additionally, we estimated the cumulative effect of metal mixtures using ENET and BKMR. ENET identified Pb and Zn as the most important predictors of preterm birth, while BMKR selected Pb, Zn, and Mn as most predictive of preterm birth. Findings from our study highlight that several metals are associated with adverse birth outcomes and stress the importance of assessing the effects of chemical mixtures on health outcomes, using multiple statistical methods and in comparison with single-pollutant models.
A few studies previously reported associations between prenatal metal mixtures and birth outcomes. Signes-Pastor et al. reported that Pb, Mn, and As combined were associated with reduced head circumference, weight, and length of newborns (Signes-Pastor et al. 2019), Lee et al. found that the joint effects of Pb and Hg were related to birthweight reduction (Lee et al. 2019), and Luo et al. confirmed a negative association between Cd and As and birthweight (Luo et al. 2017). In our study, negative effects were consistently observed for Pb in combination with other metals. Pregnant women in this Puerto Rico cohort had particularly low blood Pb concentrations (GM=0.33 μg/dL) when comparing across other studies of pregnant women, including studies conducted in Australia (median= 0.37 μg/dL)(Hinwood et al. 2013), Japan (GM=0.64 μg/dL) (Nakayama et al. 2019), Ohio, US (GM=0.7 μg/dL) (Kalloo et al. 2018), Norway (two studies: median=2.5 μg/dL and GM=0.75 μg/dL) (Birgisdottir et al. 2013; Hansen et al. 2011), South Africa (two studies: median=1.4 μg/dL and median= 2.3 μg/dL) (Mathee et al. 2014; Rudge et al. 2009) and China (median=3.2 μg/dL) (Xie et al. 2013). In addition, all blood samples in our study had Pb concentration lower than the level of concern set by CDC for pregnant women (5 μg/dL) (Ettinger and Wengrovitz 2010). However, our analysis revealed that maternal blood Pb, even at very low-levels, was the most strongly associated with increased risk of preterm birth and shorter gestational age of all the metals assessed. In recent years, concerns have also been raised that even at low levels, prenatal Pb exposure may pose toxic effects on fetal development (Anderson et al. 2016; Mushak et al. 1989; Perkins et al. 2014; Polanska et al. 2018; Silver et al. 2016; Takser et al. 2005; Taylor et al. 2017; Wu et al. 2017). Our results are consistent with these studies and provide further evidence that blood Pb at low levels, and potentially below current reference levels, may be associated with preterm birth. However, we did not find an association between Pb and birthweight, whereas several previous studies reported an inverse association with Pb and infant size when explored individually and in combination with other metals (Govarts et al. 2016; Lee et al. 2019; Luo et al. 2017).
Zn was also a key exposure associated with birth outcomes in this study. Blood concentrations of Zn (GM=4682.4 ng/mL) were similar to levels reported in previous studies of pregnant women (Callan et al. 2013; Hansen et al. 2011; Rudge et al. 2009). We found that associations between Zn and birth outcomes varied by infant sex, such that blood Zn was negatively associated with gestational age among female infants, whereas the association was not significant for male infants. It is unclear whether the infant sex-specific association we observed with Zn and gestational age could be indicative of vulnerability for women carrying a female fetus. The comparatively rapid growth of male fetuses may require more Zn compared to females, thus increased Zn may be less likely to produce adverse effects among males. Elevated blood Zn levels may also reflect the state of various processes in the body, including inflammation, oxidative stress and other key functions (Bonaventura et al. 2015; Galloway et al. 2000; Guo et al. 2011) that can play a role in gestation length.
Our negative findings with increased Zn are contrary to animal studies, observational and randomized trials on humans where maternal Zn deficiency is often associated with adverse birth outcomes, including preterm birth (Jamilian et al. 2019; Osendarp et al. 2003). Because one of many important biological functions of Zn is in the development and function of cells involved in the immune system, it is hypothesized that Zn deficiency may contribute to maternal or intrauterine infection and therefore affect premature birth (Chaffee and King 2012). Some reviews on the topic have suggested that the association observed between Zn deficiency and adverse birth outcomes could result from poor nutrition (Chaffee and King 2012; Gebreselassie and Gashe 2011; Ota et al. 2015; Wilson et al. 2016).
Mn is also an essential nutrient that plays a vital role in the body but can be toxic at excessive levels. In this population, Mn concentrations in blood (GM=11.3 ng/mL) were higher than those seen in Australian (GM=6.5 ng/mL) (Callan et al. 2013) and Norwegian pregnant women (GM=10.7ng/mL) (Hansen et al. 2011). We found that average Mn concentrations across pregnancy were associated with elevated odds of preterm birth and shorter gestational age; the association was significant for higher levels of blood Mn in the tertile analysis, indicating a potential threshold effect. BKMR graphs (Figure 3A) also showed a generally linear relationship between Mn and preterm birth at higher levels. These findings are supported by reports on high dose Mn-related maternal and fetal toxicities (Bakouei et al. 2015; Colomina et al. 1996; Sanchez et al. 1993; Vigeh et al. 2008) and the U-shaped Mn dose-response curve (Eum et al. 2014; Zota et al. 2009) observed in animal studies and epidemiologic studies.
Mechanistically, the group of metals explored in our study likely impact various biological pathways associated with preterm delivery and fetal development. One leading hypothesis of the mechanism of action is through inducing oxidative stress, defined as the homeostatic imbalance between reactive oxygen species (ROS) formation and antioxidants (Betteridge 2000). Several in vivo and in vitro studies have linked metal toxicity to the formation of ROS (Alfanie et al. 2015; Valko et al. 2005). The excessive free radical species can induce oxidative stress and cause damage to lipids, proteins and DNA in the placental tissue that eventually lead to pregnancy complications (Betteridge 2000; Martin et al. 2018; Sultana et al. 2017). Reproductive hormones also play an important role in maintaining pregnancy; in turn, disruption of the complex interplay between hormones may lead to adverse effects during gestation. A number of metals are reproductive toxicants and suspected endocrine disruptors (Bloom et al. 2010; De Coster and van Larebeke 2012; Diamanti-Kandarakis et al. 2009; Mendiola et al. 2011). Evidence suggests that metals can influence reproductive hormone levels through several pathways, including hormone synthesis, regulation, transport and metabolism, and/or interference with receptors (Garcia-Morales et al. 1994; Jurasovic et al. 2004; Meeker et al. 2010; Menke et al. 2008; Nagata et al. 2005; Telisman et al. 2007; Zeng et al. 2002; Zeng et al. 2004), with potential implications for pregnancy outcomes.
Our approaches for analyzing the effects of combined metal exposures provide evidence that Pb, Zn, and Mn are likely key exposures during pregnancy contributing to adverse birth outcomes. The weak correlations between the three metals are not likely to reflect common sources of exposure. Although BKMR analysis did not suggest interaction between the three metals, future studies constructing mechanistically based exposure mixtures are needed.
Strengths and Limitations
Our study is the first to assess the impact of metals on birth outcomes among pregnant women in Puerto Rico. The PROTECT study, a large prospective longitudinal cohort study in Puerto Rico, provided an opportunity to study the relationships between environmental pollutants and adverse birth outcomes in an at-risk population. The innovative study design allows for repeated capture of biological samples to account for the varying levels of exposures during pregnancy. Few epidemiology studies have evaluated metal exposures collectively in relation to birth outcomes, giving the proposed study a unique opportunity to test the impact of more realistic exposure profiles on birth outcomes.
The present study does have some limitations. The metal levels measured in blood depict circulating levels, which may not reflect levels in the uterine and fetal compartments that may be more biologically relevant. However, blood biomarkers may be indicative at least in part of the activity at the maternal-fetal interface, and collection of blood is much more feasible than placenta tissue or fluid samples from the uterus during pregnancy. We used the same data to calculate ERS and then to fit ERS models which has the potential for overfitting. Metal risk score needs to be validated in an independent cohort before being used as a prognostic tool by other studies. Another challenge with constructing ERS is that the ENET models assume a linear relationship between metals and the birth outcomes, and does not capture potential non-linear relationships and interactions between metals which maybe important when considering essential metals that may be toxic at high levels of exposure. However, BKMR analysis does allow for the possibility of nonlinearity and interactions between metals. There are also other statistical strategies for incorporating nonlinearity and interactions into constructing ERS that should be explored in future applications of this method (Bobb et al. 2014; Boss et al. 2018; Chipman et al. 2010; Park et al. 2017).
CONCLUSION
We considered different statistical methods to examine the effect of 10 toxic and essential trace metal(loid)s and metal risk score on various birth outcomes among pregnant women in Northern Puerto Rico. Although the PROTECT cohort has lower Pb concentrations (GM=0.33 μg/dL) compared to the mainland US and other studies of pregnant women in different countries, our findings suggest that low-level prenatal Pb exposure, as well as elevated Mn and Zn exposure, may adversely affect birth outcomes. These findings provide further support for the need to reduce Pb exposure as much as possible among pregnant women. Improved understanding of environmental and other factors that contribute to preterm birth, together with developing sustainable technologies to remove contamination, will have a direct public health impact in Puerto Rico.
Supplementary Material
Highlights.
First study to assess maternal metals in relation to birth outcomes in Puerto Rico
Highlights the importance of assessing the effects of mixtures on health outcomes
Pb, even at low-levels, was the most strongly associated with risk of preterm birth
Elevated Mn and Zn exposure may adversely affect birth outcomes
Acknowledgements
We would like to extend our gratitude to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the Delta OBGyn Group in Manati, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo.
Funding
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS grant number P30ES017885 and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCE
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Cordero JF, et al. 2019. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern puerto rico. Environ Res 169:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfanie I, Muhyi R, Suhartono E. 2015. Effect of heavy metal on malondialdehyde and advanced oxidation protein products cencentration a focus on arsenic, cadmium, and mercury. Journal of Medical and Bioengineering Vol 4. [Google Scholar]
- Anderson DW, Mettil W, Schneider JS. 2016. Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicol Lett 246:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KW, Savitz DA, Hertz-Picciotto I. 1994. Prenatal lead exposure in relation to gestational age and birth weight: A review of epidemiologic studies. Am J Ind Med 26:13–32. [DOI] [PubMed] [Google Scholar]
- Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, et al. 2018. Maternal and cord blood manganese (mn) levels and birth weight: The mirec birth cohort study. Int J Hyg Environ Health 221:876–882. [DOI] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, et al. 2018. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in northern puerto rico: Predictors and trends. Environ Int 121:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, et al. 2020. Predictors of urinary and blood metal(loid) concentrations among pregnant women in northern puerto rico. Environ Res 183:109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouei S, Reisian F, Lamyian M, Haji Zadeh E, Zamanian H, Taheri Kharameh Z. 2015. High intake of manganese during second trimester, increases the risk of preterm delivery: A large scale cohort study. Glob J Health Sci 7:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. 1989. Weight in infancy and death from ischaemic heart disease. Lancet 2:577–580. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57:289–300. [Google Scholar]
- Berkowitz G, Lapinski R, Wolff M. 1996. The role of dde and polychlorinated biphenyl levels in preterm birth. Archives of environmental contamination and toxicology 30:139–141. [DOI] [PubMed] [Google Scholar]
- Betteridge DJ. 2000. What is oxidative stress? Metabolism 49:3–8. [DOI] [PubMed] [Google Scholar]
- Birgisdottir BE, Knutsen HK, Haugen M, Gjelstad IM, Jenssen MT, Ellingsen DG, et al. 2013. Essential and toxic element concentrations in blood and urine and their associations with diet: Results from a norwegian population study including high-consumers of seafood and game. Sci Total Environ 463–464:836–844. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. 2013. Born too soon: The global epidemiology of 15 million preterm births. Reprod Health 10 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Parsons PJ, Steuerwald AJ, Schisterman EF, Browne RW, Kim K, et al. 2010. Toxic trace metals and human oocytes during in vitro fertilization (ivf). Reprod Toxicol 29:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2014. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Henn BC, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via bayesian kernel machine regression. Environmental Health 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura P, Benedetti G, Albarède F, Miossec P. 2015. Zinc and its role in immunity and inflammation. Autoimmunity reviews 14:277–285. [DOI] [PubMed] [Google Scholar]
- Boss J, Zhai J, Aung MT, Ferguson KK, Johns LE, McElrath TF, et al. 2018. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: A time to event analysis using summative phthalate risk scores. Environmental Health 17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan AC, Hinwood AL, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. 2013. Maternal exposure to metals--concentrations and predictors of exposure. Environ Res 126:111–117. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environ Int 62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Graziano A, Lo Monte G, Bordi G, Moscarini M. 2013. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17:2198–2206. [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. 2018. Fourth national report on human exposure to environmental chemicals updated tables.
- Centers for Disease Control and Prevention. 2009. Fourth report on human exposure to environmental chemicals. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention. 2012. Increasing prevalence of diagnosed diabetes--united states and puerto rico, 1995–2010. MMWR Morb Mortal Wkly Rep 61:918–921. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2019. Fourth national report on human exposure to environmental chemicals updated tables.
- Chaffee BW, King JC. 2012. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr Perinat Epidemiol 26 Suppl 1:118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li Y, Zhang B, Zhou A, Zheng T, Huang Z, et al. 2018. Maternal exposure to nickel in relation to preterm delivery. Chemosphere 193:1157–1163. [DOI] [PubMed] [Google Scholar]
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman HA, George EI, McCulloch RE. 2010. Bart: Bayesian additive regression trees. The Annals of Applied Statistics 4:266–298. [Google Scholar]
- Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, et al. 2018. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the vhembe south african birth cohort. Environ Int 113:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomina MT, Domingo JL, Llobet JM, Corbella J. 1996. Effect of day of exposure on the developmental toxicity of manganese in mice. Vet Hum Toxicol 38:7–9. [PubMed] [Google Scholar]
- Committee on Obstetric Practice tAIoUiM, and, Medicine; tSfM-F. 2017. Committee opinion no 700: Methods for estimating the due date.
- De Coster S, van Larebeke N. 2012. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J Environ Public Health 2012:713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev 30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AS, Wengrovitz AM. 2010. Guidelines for the identification and management of lead exposure in pregnant and lactating women.
- Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, Hong YC, et al. 2014. Maternal blood manganese level and birth weight: A moceh birth cohort study. Environ Health 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, O’Neill MS, Meeker JD. 2013. Environmental contaminant exposures and preterm birth: A comprehensive review. Journal of Toxicology and Environmental Health, Part B 16:69–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in puerto rico. Environmental science & technology 48:7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD. 2016. Repeated measures analysis of associations between urinary bisphenol-a concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod Toxicol 66:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. 2019. Environmental phthalate exposure and preterm birth in the protect birth cohort. Environ Int 132:105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SP, McMillan DC, Sattar N. 2000. Effect of the inflammatory response on trace element and vitamin status. Annals of clinical biochemistry 37:289–297. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, Gottardis MM, et al. 1994. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem 269:16896–16901. [PubMed] [Google Scholar]
- Garza JR, Perez EA, Prelip M, McCarthy WJ, Feldman JM, Canino G, et al. 2011. Occurrence and correlates of overweight and obesity among island puerto rican youth. Ethn Dis 21:163–169. [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie SG, Gashe FE. 2011. A systematic review of effect of prenatal zinc supplementation on birthweight: Meta-analysis of 17 randomized controlled trials. J Health Popul Nutr 29:134–140. [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, et al. 2016. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin IJ. 2014. Perinatal growth and nutrition:CRC Press.
- Guo C-H, Chen P-C, Yeh M-S, Hsiung D-Y, Wang C-L. 2011. Cu/zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clinical biochemistry 44:275–280. [DOI] [PubMed] [Google Scholar]
- Hansen S, Nieboer E, Sandanger TM, Wilsgaard T, Thomassen Y, Veyhe AS, et al. 2011. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J Environ Monit 13:2143–2152. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, Callan AC, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. 2013. Cadmium, lead and mercury exposure in non smoking pregnant women. Environ Res 126:118–124. [DOI] [PubMed] [Google Scholar]
- Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, et al. 2019. The effects of magnesium-zinc-calcium-vitamin d co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 19:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. 2004. Semen quality and reproductive endocrine function with regard to blood cadmium in croatian male subjects. Biometals 17:735–743. [DOI] [PubMed] [Google Scholar]
- Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Karagas M, et al. 2018. Profiles and predictors of environmental chemical mixture exposure among pregnant women: The health outcomes and measures of the environment study. Environ Sci Technol 52:10104–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Meeker JD, Carroll R, Zhao S, Mourgas MJ, Richards MJ, et al. 2018. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int 121:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Platt RW, Wen SW, Joseph K, Allen A, Abrahamowicz M, et al. 2001. A new and improved population-based canadian reference for birth weight for gestational age. Pediatrics 108:e35–e35. [DOI] [PubMed] [Google Scholar]
- Lee S, Hong YC, Park H, Kim Y, Ha M, Ha E. 2019. Combined effects of multiple prenatal exposure to pollutants on birth weight: The mothers and children’s environmental health (moceh) study. Environ Res:108832. [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. 2012. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. [DOI] [PubMed] [Google Scholar]
- Luo Y, McCullough LE, Tzeng JY, Darrah T, Vengosh A, Maguire RL, et al. 2017. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health 17:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Katz SL, Leeson P, Thebaud B, Nuyt AM. 2016. Preterm birth: Risk factor for early-onset chronic diseases. CMAJ 188:736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss III JF. 2004. A polymorphism in the promoter region of tnf and bacterial vaginosis: Preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. American journal of obstetrics and gynecology 190:1504–1508. [DOI] [PubMed] [Google Scholar]
- Marlow N, Wolke D, Bracewell MA, Samara M, Group EPS. 2005. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 352:9–19. [DOI] [PubMed] [Google Scholar]
- Martin A, Faes C, Debevec T, Rytz C, Millet G, Pialoux V. 2018. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol 17:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathee A, Naicker N, Kootbodien T, Mahuma T, Nkomo P, Naik I, et al. 2014. A cross-sectional analytical study of geophagia practices and blood metal concentrations in pregnant women in johannesburg, south africa. S Afr Med J 104:568–573. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Padmanahban V, Diamond MP, Puscheck E, et al. 2010. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil Steril 93:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ Sci Technol 47:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Moreno JM, Roca M, Vergara-Juarez N, Martinez-Garcia MJ, Garcia-Sanchez A, et al. 2011. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: A pilot study. Environ Health 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Guallar E, Shiels MS, Rohrmann S, Basaria S, Rifai N, et al. 2008. The association of urinary cadmium with sex steroid hormone concentrations in a general population sample of us adult men. Bmc Public Health 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelson CK, Troisi J, LaLonde A, Symes SJK, Thurston SW, DiRe LM, et al. 2018. Placental concentrations of essential, toxic, and understudied metals and relationships with birth outcomes in chattanooga, tn. Environ Res 168:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. 2007. Epidemiology and environmental factors in preterm labour. Best Pract Res Clin Obstet Gynaecol 21:773–789. [DOI] [PubMed] [Google Scholar]
- Mushak P, Davis JM, Crocetti AF, Grant LD. 1989. Prenatal and postnatal effects of low-level lead exposure: Integrated summary of a report to the u.S. Congress on childhood lead poisoning. Environ Res 50:11–36. [DOI] [PubMed] [Google Scholar]
- Nagata C, Nagao Y, Shibuya C, Kashiki Y, Shimizu H. 2005. Urinary cadmium and serum levels of estrogens and androgens in postmenopausal japanese women. Cancer Epidemiol Biomarkers Prev 14:705–708. [DOI] [PubMed] [Google Scholar]
- Nakayama SF, Iwai-Shimada M, Oguri T, Isobe T, Takeuchi A, Kobayashi Y, et al. 2019. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: The japan environment and children’s study (jecs). J Expo Sci Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osendarp SJ, West CE, Black RE, Maternal Zinc Supplementation Study G. 2003. The need for maternal zinc supplementation in developing countries: An unresolved issue. J Nutr 133:817S–827S. [DOI] [PubMed] [Google Scholar]
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. 2015. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev:CD000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Gonzalez M, Garcia-Fragoso L. 2008. Prevalence of overweight and obesity in a group of children between the ages of 2 to 12 years old in puerto rico. P R Health Sci J 27:159–161. [PubMed] [Google Scholar]
- Park SK, Zhao Z, Mukherjee B. 2017. Construction of environmental risk score beyond standard linear models using machine learning methods: Application to metal mixtures, oxidative stress and cardiovascular disease in nhanes. Environ Health 16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CM, Guzman M, Ortiz AP, Estrella M, Valle Y, Perez N, et al. 2008. Prevalence of the metabolic syndrome in san juan, puerto rico. Ethn Dis 18:434–441. [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. 2014. Very low maternal lead level in pregnancy and birth outcomes in an eastern massachusetts population. Ann Epidemiol 24:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Pawlas N, Wesolowska E, Jankowska A, Jagodic M, et al. 2018. Sex-dependent impact of low-level lead exposure during prenatal period on child psychomotor functions. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR. 2016. Placental metal concentrations in relation to maternal and infant toenails in a u.S. Cohort. Environ Sci Technol 50:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Soto WT, Rodriguez-Figueroa L, Calderon G. 2010. Prevalence of childhood obesity in a representative sample of elementary school children in puerto rico by socio-demographic characteristics, 2008. P R Health Sci J 29:357–363. [PubMed] [Google Scholar]
- Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. 2009. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of south african delivering women. J Environ Monit 11:1322–1330. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Domingo JL, Llobet JM, Keen CL. 1993. Maternal and developmental toxicity of manganese in the mouse. Toxicol Lett 69:45–52. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, et al. 2019. Prenatal exposure to metal mixture and sex-specific birth outcomes in the new hampshire birth cohort study. Environ Epidemiol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MK, Li X, Liu Y, Li M, Mai X, Kaciroti N, et al. 2016. Low-level prenatal lead exposure and infant sensory function. Environ Health 15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. 2017. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol 77. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Lafond J. 2005. Very low level environmental exposure to lead and prolactin levels during pregnancy. Neurotoxicol Teratol 27:505–508. [DOI] [PubMed] [Google Scholar]
- Tardiff RG, Carson ML, Ginevan ME. 2006. Updated weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products. Regulatory Toxicology and Pharmacology 45:185–205. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Kordas K, Golding J, Emond AM. 2017. Effects of low-level prenatal lead exposure on child iq at 4 and 8 years in a uk birth cohort study. Neurotoxicology 62:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P. 2007. Reproductive toxicity of low-level lead exposure in men. Environ Res 105:256–266. [DOI] [PubMed] [Google Scholar]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. 2015. Metals exposure and risk of small-for-gestational age birth in a canadian birth cohort: The mirec study. Environmental Research 140:430–439. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin M. 2005. Metals, toxicity and oxidative stress. Current medicinal chemistry 12:1161–1208. [DOI] [PubMed] [Google Scholar]
- Vigeh M, Yokoyama K, Ramezanzadeh F, Dahaghin M, Fakhriazad E, Seyedaghamiri Z, et al. 2008. Blood manganese concentrations and intrauterine growth restriction. Reprod Toxicol 25:219–223. [DOI] [PubMed] [Google Scholar]
- Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. 2014. International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the intergrowth-21st project. The Lancet 384:857–868. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. 2015. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in puerto rico. Int J Hyg Environ Health 218:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Walker M, Wade MG, Liu S, Krewski D. 2007. Environmental hazards: Evidence for effects on child health. J Toxicol Environ Health B Crit Rev 10:3–39. [DOI] [PubMed] [Google Scholar]
- Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. 2016. Association between maternal zinc status, dietary zinc intake and pregnancy complications: A systematic review. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham G, Fenster L. 2008. Environmental contaminants and pregnancy outcomes. Fertility and sterility 89:e111–e116. [DOI] [PubMed] [Google Scholar]
- Wu S, Hivert MF, Cardenas A, Zhong J, Rifas-Shiman SL, Agha G, et al. 2017. Exposure to low levels of lead in utero and umbilical cord blood DNA methylation in project viva: An epigenome-wide association study. Environ Health Perspect 125:087019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ding G, Cui C, Chen L, Gao Y, Zhou Y, et al. 2013. The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut 175:30–34. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Fall CH, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, et al. 1995. Fetal growth and glucose and insulin metabolism in four-year-old indian children. Diabet Med 12:330–336. [DOI] [PubMed] [Google Scholar]
- Zeng X, Lin T, Zhou Y, Kong Q. 2002. Alterations of serum hormone levels in male workers occupationally exposed to cadmium. J Toxicol Environ Health A 65:513–521. [DOI] [PubMed] [Google Scholar]
- Zeng X, Jin T, Buchet JP, Jiang X, Kong Q, Ye T, et al. 2004. Impact of cadmium exposure on male sex hormones: A population-based study in china. Environ Res 96:338–344. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, et al. 2019. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ Int 123:325–336. [DOI] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. 2009. Maternal blood manganese levels and infant birth weight. Epidemiology 20:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H 2006. The adaptive lasso and its oracle properties. Journal of the American statistical association 101:1418–1429. [Google Scholar]
- Zou H, Zhang HH. 2009. On the adaptive elastic-net with a diverging number of parameters. Ann Stat 37:1733–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


