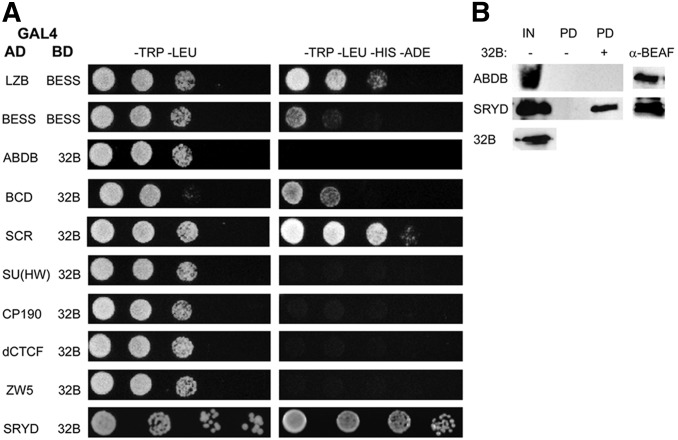
Figure 1.
Y2H and pull-down tests for interactions between BEAF-32B and specific proteins. (A) BEAF-32B was fused to the C-terminal end of the GAL4 DNA-BD, and candidate proteins were fused to the C-terminal end of the GAL4-AD for use in Y2H assays. Interactions of the BEAF BESS domain with itself and the LZB domain were used as positive controls (see Figure 2A). As previously reported, the interaction of the BESS domain with itself was weaker than its interaction with the LZB (Avva and Hart 2016). Serial fivefold dilutions of OD600 0.1 yeast were spotted onto plates. Left panels (−TRP –LEU) show growth on plates selecting for plasmids. Right panels (−TRP –LEU –HIS –ADE) show growth on plates additionally selecting for reporter gene expression. Shown are proteins from Table 1 that interact with 32B, insulator proteins that do not interact with 32B as examples of negative results, and interaction with Sry-δ from the cDNA library screen. (B) Bacterial protein extracts containing N-terminal FLAG-tagged 32B and N-terminal Myc-tagged transcription factors were mixed and pulled down using anti-FLAG M2 beads. After SDS-PAGE, proteins were detected using anti-Myc or anti-BEAF antibodies. Sry-δ was pulled down only in the presence of FLAG-32B, while the negative control Myc-Abd-B was not pulled down. α-BEAF: detection of pulled down FLAG-32B from the (+) lanes (25% of pulldown); AD, activation domain; BD, binding domain; BEAF, Boundary Element-Associated Factor; cDNA, complementary DNA; IN, input proteins (20% of input); LZB, leucine zipper plus BESS; PD, proteins pulled down in the absence (−) or presence (+) of FLAG-32B (25% of pulldown); Sry-δ, Serendipity δ; Y2H, yeast two-hybrid.