Figure 2: Development of Tolerogenic Immune-Modifying Nanoparticles encapsulating gliadin (TIMP-GLIA) (II).
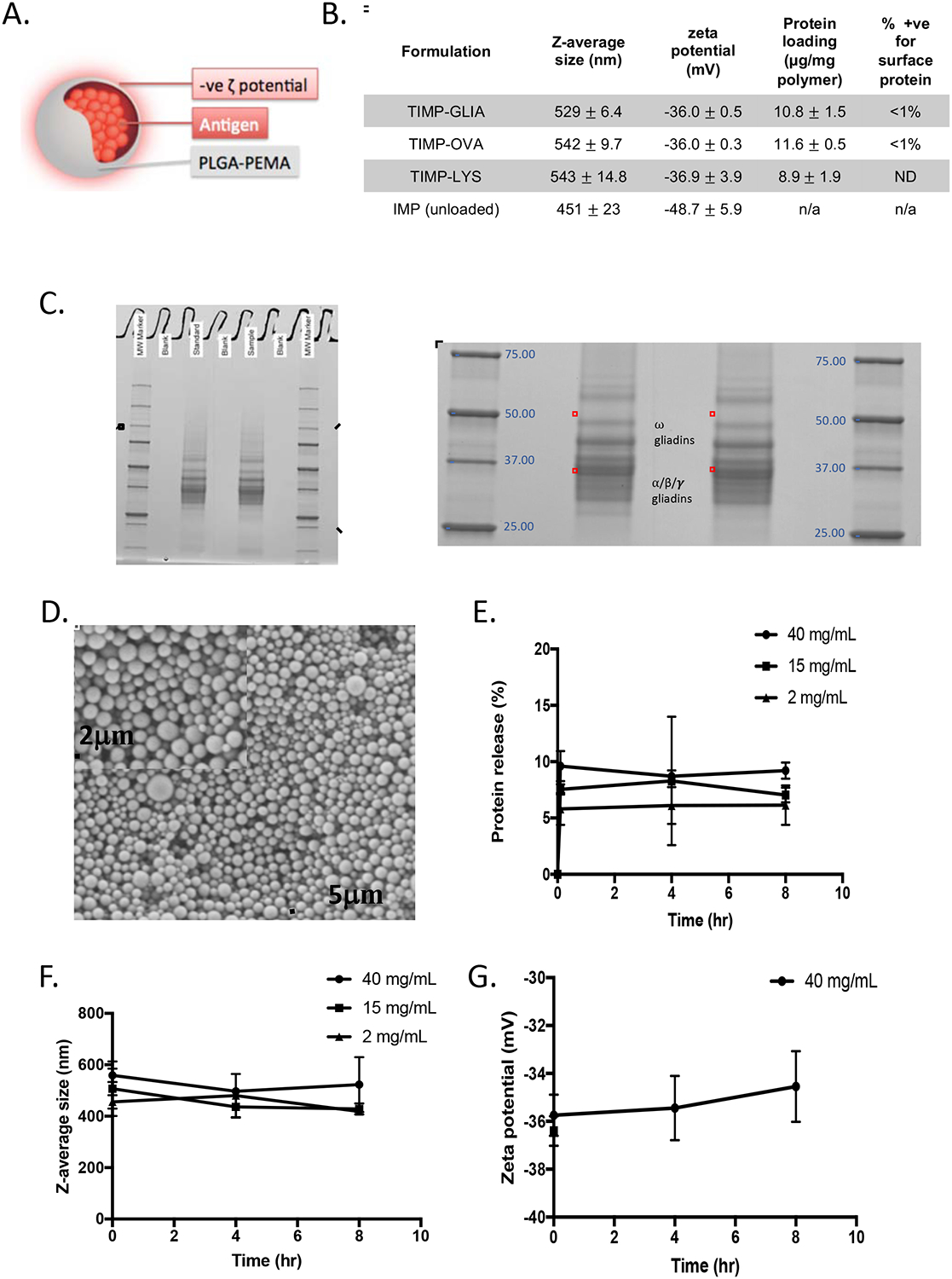
. A) Schematic representation of TIMP. B) Four different formulations of PLGA-PEMA nanoparticles were prepared for testing, encapsulating either gliadin (TIMP-GLIA), ovalbumin (TIMP-OVA) or lysozyme (TIMP-LYS), or remaining unloaded (IMP). Size, charge, protein loading (mean +/− SD) and percentage of particles positive for surface protein (FACS) were analyzed. C) SDS-PAGE of gliadin preparation used for production of TIMP-GLIA (duplicates, central rows). D) Scanning electron microscopy of a representative TIMP-GLIA suspension. E-G) Analysis of TIMP-GLIA stability in water over 8h, measuring protein release (E), size (F) and charge (G; triplicates, mean +/− SD).
