Figure 6. TIMP-GLIA clearance, and interaction with human peripheral blood mononuclear cells (PBMC).
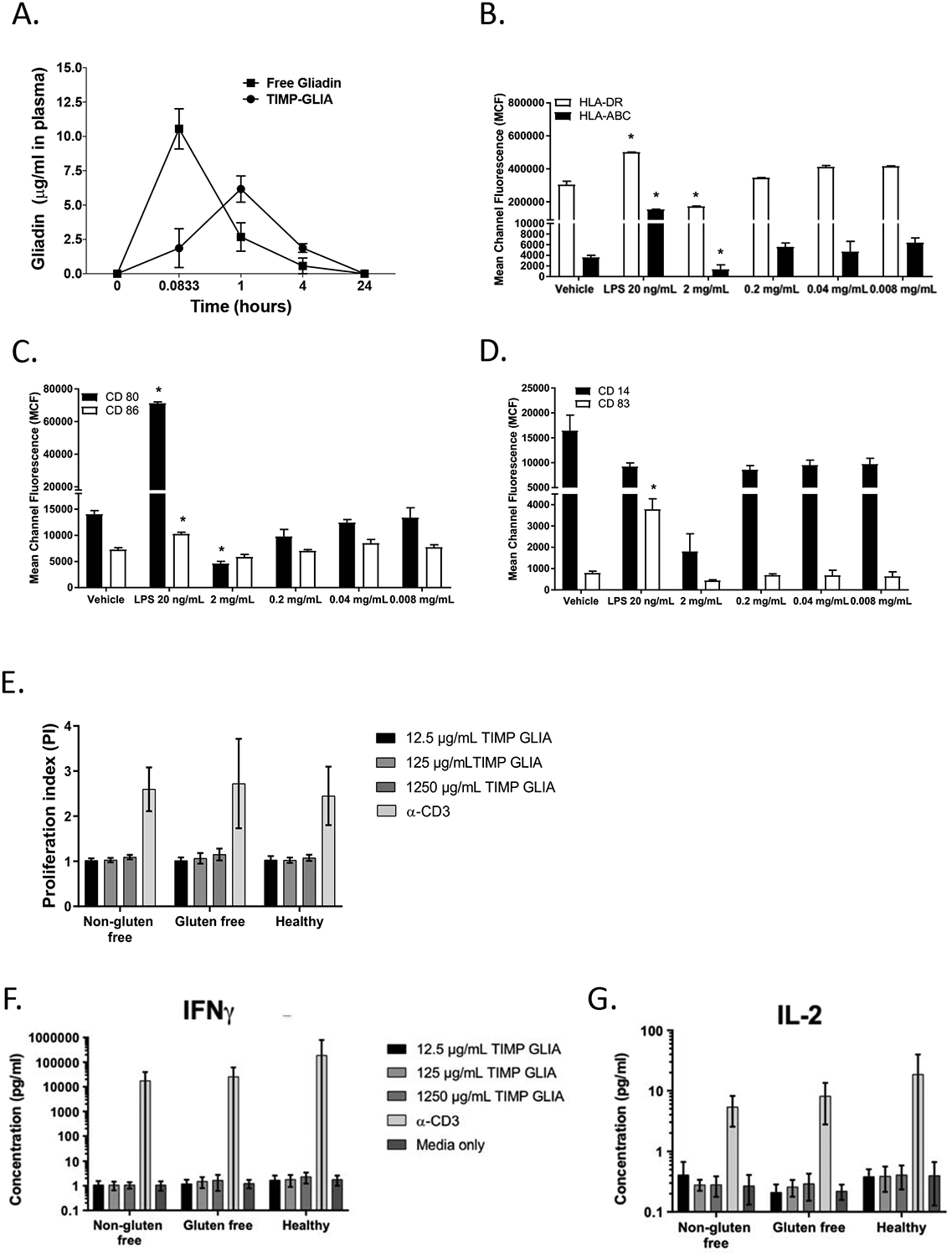
A) Naïve C57BL/6 mice (n=3 per time point) were injected intravenously with either 2.5mg of TIMP-GLIA, or 40ug of free gliadin (corresponding amount). Mice were bled at 5min, 1h, 4h, and 24h. Collected plasma samples were assessed for the level of free gliadin (ELISA; mean concentration +/− SEM). B-D) Immature dendritic cells derived from human PBMC (n=6–9) were treated with vehicle (PBS), LPS 20 ng/mL (positive control) or TIMP-GLIA at increasing concentrations for 48 hours. Surface expression of HLA-ABC and HLA-DR (B), CD80 and CD86 (C) and CD14 and CD83 (D) were determined by flow cytometry (mean channel fluorescence; mean ± SD). E-G) Human PBMC from celiac disease patients on normal or gluten free diet, or healthy controls, were stimulated with anti-CD3 antibody (positive control) or TIMP-GLIA at increasing concentrations (triplicates; n=9–11). Proliferation (E), and IFNG (F) or IL2 (G) cytokine secretion were measured after 72h. Data is expressed as proliferation index (relating to unstimulated cells; luminescent cell viability assay), or cytokine concentrations (V-Plex assay). Statistical analyses were performed using paired t-test, compared to vehicle group (B-D; *p≤0.05).
