Figure 1.
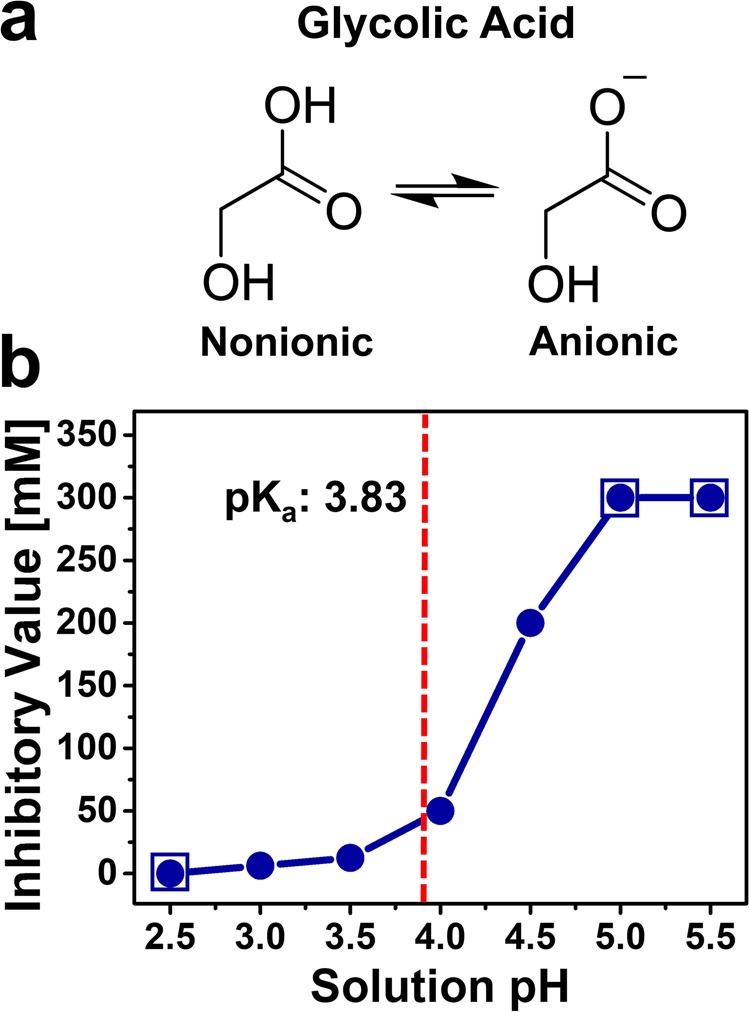
Evaluation of glycolic acid as an antibacterial agent to inhibit C. acnes viability. (a) Molecular structure of glycolic acid in the nonionic (protonated) and anionic (deprotonated) states. The equilibrium ratio of glycolic acid molecules in the two states depends on the pH condition. (b) Experimentally determined lowest concentration of glycolic acid to fully inhibit C. acnes viability in different pH conditions. The C. acnes cell concentration was 1 × 106 CFU mL−1 and cell suspensions were incubated in glycolic acid solutions at different pH conditions for 1 hr before agar plating to determine if glycolic acid treatment inhibited cell viability. Each data point is representative of three independent experiments. The boxed-in circles indicate pH conditions where an inhibitory concentration was not recorded for one of two reasons: the pH condition itself during the incubation step caused loss of C. acnes viability (pH 2.5) or glycolic acid was inactive (i.e., not antibacterial) within the test range up to 200 mM (pH 5.0 and 5.5). The dashed vertical line represents the pKa value of glycolic acid, which is around pH 3.83.
