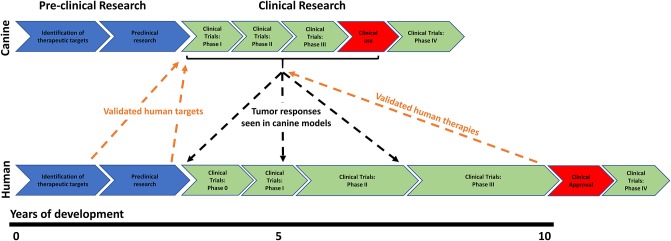
Figure 1.
Schematic diagram outlining the integration of human and canine research programmes to improve current drug development strategies. The typical course of human drug research and development is very linear, progressing through in vitro target identification and validation right through to phase 0, I, II, III, and IV clinical trials. Unfortunately, most drugs which show promise in pre-clinical studies subsequently fail to show efficacy in clinical trials. At this point a substantial amount of time and money has been spent, which can ultimately deter drug companies from developing new therapeutics. The integration of translational canine studies into human drug development programmes could help identify drug failures sooner or allow for drug refinement prior to human clinical studies. Shorter disease-free progression times seen in dogs also allows for rapid conclusion of the clinical trials that can incorporate assessment of drug activity, toxicity, pharmacokinetics, pharmacodynamics, dose regimens, combination therapies and histology.