Figure 2.
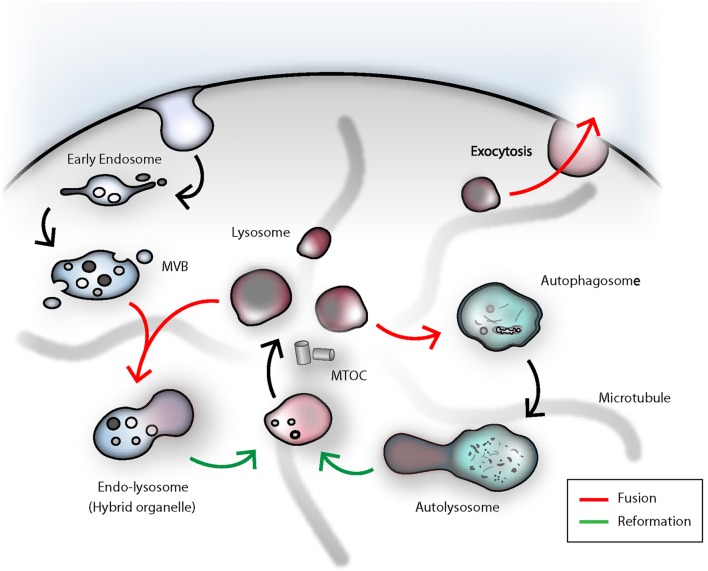
The dense-core secretory lysosomes are the terminal storage unit for acidic hydrolases and effector molecules in NK cells. The lysosome as such is a highly dynamic compartment and can engage in several fusion events in order to deliver its degradative capacity for endocytosis, autophagy or killing of target cells by NK cells. Termination of signal transduction is an important task for the orchestration of NK cell effector functions. Early endosomal cargo can be propagated by gradual-maturation of an early endosome to a late endosome, which finally can fuse with a lysosome for degradation by acidic hydrolases. As a result, an endolysosomal hybrid organelle is formed. Lysosomes can be reformed through tubulation from the latter compartment and leads to formation of a proto-lysosome, which undergoes further maturation and content condensation. In analogy to that, lysosomes can also engage in autophagy. In immune cells, autophagy fulfills the important task of eradicating worn-out mitochondria. Damaged organelles or proteins can be entrapped in autophagosomes, ultimately fusing with lysosomes. Upon termination of autophagy, again, through tubulation and membrane extrusions, consumed proto-lysosomes are regenerated and mature to lysosomes. During target cell killing, NK cells go through step-wise receptor cross-linking, granule polarization and eventually lysosome-related organelle fusion with the plasma membrane at the immunological synapse. During this process, the effector molecules and the electron-dense core can be ejected and the lysosomal membrane will be recovered through endocytosis. MVB, multi-vesicular body; MTOC, microtubule-organizing center.