Abstract
Dietary energy density (DED) has been widely considered a risk factor for weight gain. In adolescents, however, the evidence is inconclusive, and in Mexico, the ways in which DED is associated with overweight and obesity are unknown. Our study analysed the association of DED with overweight or obesity (OW‐O) in Mexican adolescents included in the National Health and Nutrition Survey 2012 (ENSANUT 2012). We analysed the data from a 7‐day Food Frequency Questionnaire administered to 2,203 Mexican adolescents aged 12–19 years. DED was calculated excluding all beverages. Plausible and implausible reporters were identified based on the relationship between the reported energy intake and the estimated energy requirement. The association of DED with body mass index (BMI)‐for‐age and OW‐O was analysed using multivariate statistical models restricted to plausible reporters. The combined prevalence of overweight and obesity was 35.4% in the complete sample and 27.8% in the sample of plausible reporters. Mean DED was 177 kcal/100 g, with higher DED in the north of the country. The proportion of plausible reporters was 38.5%. We found a positive association between high DED and the BMI‐for‐age z‐score (β = 0.347; 95% CI [0.101, 0.594]; P = 0.006), controlling for sociodemographic and dietary variables, but no significant association with OW‐O. It is necessary to consider the DED in the design and implementation of strategies to reduce energy density in the diets of young Mexicans.
Keywords: adolescents, diet, dietary energy density, Mexico, nutrition surveys, overweight and obesity
Key messages.
One out of every five Mexican adolescents is overweight, and one out of every 10 is obese.
The weight gain in Mexican population is related to the excessive consumption of high‐energy density foods that usually are high‐saturated‐fat and/or added‐sugar products. The contribution of these products is 20% of total energy intake in Mexican adolescents.
Evidence suggests a positive association between dietary energy density and body mass index (BMI) in children and adults; however, results in adolescents are inconclusive.
This is the first research effort in Mexico to identify the association of dietary energy density and BMI‐for‐age with the risk of overweight and obesity in adolescents and serves as a basis for issuing evidence‐based recommendations on the design of weight‐related interventions.
1. INTRODUCTION
Overweight and obesity (OW‐O) are considered a global epidemic and a major national public health issue of increasing concern (Rivera et al., 2014). In recent decades, Mexico has experienced a dramatic increase in the prevalence of OW‐O among all populations (Barquera, Campos, & Rivera, 2013; Gutiérrez et al., 2012), with a particularly pronounced impact observed in children and adolescents (Hernández‐Cordero, Cuevas‐Nasu, Morán‐Ruán, Méndez‐Gómez Humarán, & Ávila‐Arcos, 2017). This could contribute to the high risk presented by the Mexican population for chronic diseases such as type 2 diabetes, coronary heart disease (Rocchini, 2011), metabolic syndrome, cancer, and a variety of psychological and social problems in childhood and thereafter (Gurnani, Birken, & Hamilton, 2015). According to the 2012 National Health and Nutrition Survey (ENSANUT 2012), 35% of Mexican adolescents were overweight or obese (Gutiérrez et al., 2012). This reflects an increase of 24.7 percentage points in the combined prevalence of OW‐O from 1988 to 2012 (Gutiérrez et al., 2012; Hernández‐Cordero et al., 2017) and, therefore, a trend towards weight gain.
As stated by the World Health Organization (WHO, 2003), diet is a fundamental risk factor for OW‐O. More specifically, dietary energy density (DED) has been associated with weight gain (Pérez‐Escamilla et al., 2012). DED is defined as “the amount of energy in food per unit of weight” (Pérez‐Escamilla et al., 2012; Rolls, 2009). It is generally expressed in kilocalories per 100 g of food (Johnson, Wilks, Lindroos, & Jebb, 2009a; O'Connor, Walton, & Flynn, 2013) and can serve as an indicator for the nutritional quality of diets depending on whether it has a high‐ or low‐energy content (O'Connor et al., 2013). According to the World Cancer Research Fund (WCRF), high‐energy dense foods have an energy content greater than 225–275 kcal/100 g, such as fast foods, snacks, confections, candies, and pastries including cakes and biscuits, (WCRF/American Institute for Cancer Research [AICR], 2007), which are usually high‐saturated‐fat and/or added‐sugar (HSFAS) products (Aburto, Pedraza, Sánchez, Batis, & Rivera, 2016). In Mexico, diet accounts for approximately 10% of the burden of disease (Gómez‐Dantés et al., 2016) and is marked by an excessive consumption of sugar‐sweetened beverages (SSBs; Sánchez, Batis, Lutter, & Rivera, 2016) and HSFAS products (Aburto et al., 2016; López‐Olmedo et al., 2016).
Several studies suggest that DED is associated with OW‐O (Pérez‐Escamilla et al., 2012; Rouhani, Haghighatdoost, Surkan, & Azadbakht, 2016). A positive association between DED and increased body mass index (BMI) has been observed in adults (Ledikwe et al., 2006; Mendoza, Drewnowski, & Christakis, 2007), and low DED diets have been found to exert a significant protective effect against abdominal obesity (Du et al., 2009). In children, there is consistent evidence of an association between DED and weight gain based on measurements such as the fat mass index (Johnson, Mander, Jones, Emmett, & Jebb, 2008a, 2008b; Pérez‐Escamilla et al., 2012) and the BMI z‐score (Johnson et al., 2008a; Vernarelli, Mitchell, Hartman, & Rolls, 2011). Although the mechanisms of how DED affects weight gain are not well understood, evidence suggests that it is related to the energy compensation capacity in this age group (Rolls, 2009; Leahy, Birch, & Rolls, 2008; Birch & Deysher, 1986). Despite the confirmed role of DED in weight gain among children and adults (Pérez‐Escamilla et al., 2012; Rouhani et al., 2016), data on the frequency of its occurrence in adolescents are limited, with results remaining as yet inconclusive (Van Sluijs et al., 2016) and even contradictory (Alexy, Sichert‐Hellert, Kersting, & Schultze‐Pawlitschko, 2004; McCaffrey et al., 2008; Murakami, Miyake, Sasaki, Tanaka, & Arakawa, 2012a). In Mexico, studies on DED are scarce, and the way in which it is associated with OW‐O in the adolescent population is unknown. Evidence clearly shows, however, that consumption of HSFAS products has been increasing (Aburto et al., 2016; López‐Olmedo et al., 2016) in proportion to the high prevalence of OW‐O (Hernández‐Cordero et al., 2017).
Identifying the association of DED with weight status in this age group would provide useful information for designing obesity interventions focused on youth. The objective of our research was to analyse the association between DED and OW‐O in Mexican adolescents based on data from ENSANUT 2012.
2. METHODS
The ENSANUT was conducted in Mexico between October 2011 and May 2012. It is a probabilistic national survey with multistage, stratified, and cluster sampling. The detailed description of sampling procedures and survey methodology is described elsewhere (Romero‐Martínez et al., 2013).
2.1. Study population
ENSANUT 2012 collected data from a subsample of the total adolescent population, consisting of 2,203 subjects aged 12 to 19 years, who were able to provide anthropometric and dietary information. Pregnant and lactating women (n = 72) as well as subjects with insufficient or implausible anthropometric data (n = 87) were excluded from analysis. Only plausible BMI‐for‐age z‐score values between −5 and +5 standard deviation (SD) were considered. Analysis was performed on a final sample of 2,044 adolescents representing an expanded pool of 21,337,591 Mexican adolescents.
It is important to mention that only 1,850 respondents provided information concerning screen time, representing an adjusted sample of 19,135,744 adolescents; the others did not answer the corresponding item from the questionnaire.
2.2. Description of variables
2.2.1. Anthropometric measurements
Anthropometric measurements were taken by standardized, trained personnel (Habitch, 1974; Shamah‐Levy, Villalpando‐Hernández, & Rivera‐Dommarco, 2006). Data on weight were obtained using Seca digital scales, model 872, with 0.1‐kg precision; height was measured using stadiometers with 0.1‐cm precision. The BMI‐for‐age z‐score (kg/m2) was calculated following the WHO Reference 2007 (De Onis et al., 2007). Adolescents with z‐scores between >1 SD and ≤2 SD were classified as overweight, whereas those above >2 SD were considered obese.
2.2.2. Dietary information
We used a previously validated 7‐day Food Frequency Questionnaire (FFQ; Denova‐Gutiérrez et al., 2016) including 167 items classified into 14 food groups; of total food items, 40 were beverages. Adolescents were asked to describe their consumption of specific items: The questions concerned portions and frequency including daily recurrence as well as the number of days and times per week.
The National Institute of Public Health Food Composition Database was used to further determine energy, nutrients, and fibre consumption (Nutrient Database Compilation elaborated by the National Institute of Public Health, unpublished material). Detailed information is found elsewhere (Ramírez‐Silva et al., 2016).
2.2.3. Dietary energy density
Calculation of DED involves a particularly complex decision regarding the inclusion (or exclusion) of beverages (Johnson et al., 2009a; O'Connor et al., 2013). For this study, we included solid foods only, because the inclusion of beverages increases the variability of DED and has differing effects on energy intake (EI; Johnson et al., 2009a). DED was calculated by dividing the total energy of all food (that are not beverages) consumed (kcal) by the total weight of food (g; not beverages), multiplied by 100 (kcal/100 g). Foods that are not beverages included some preparations such as ice cream, jellies and soups. All liquid foods, broths (without meat or vegetables), and dairy and nondairy drinks with and without sugar were excluded, as was sugar added to beverages such as tea, coffee, milk, or chocolate.
2.2.4. Sugar‐sweetened beverages
The SSB group included soft drinks, natural juices with and without sugar, industrialized beverages, nectars, alcoholic beverages, natural water with added sugar, yogurt, maize with water or milk, tea, coffee, and sugar added to tea or coffee.
2.2.5. Sociodemographic variables
Demographic and socio‐economic characteristics of sampled individuals such as sex, age, area, region, and socio‐economic status (SES) were obtained through the household questionnaire. The country was divided into three geographic regions (North, Centre and South) and into urban and rural areas (localities comprised ≥2,500 or <2,500 inhabitants, respectively; Gutiérrez et al., 2012). For the construction and classification of the SES, household characteristics were determined according to a principal components factor analysis previously used in other national nutrition surveys in Mexico (Gutiérrez et al., 2012; Olaiz‐Fernández et al., 2006) and were categorized into tertiles: low, medium, and high. Calculation is described elsewhere (Gutiérrez, 2013).
2.2.6. Screen time
This variable was defined as the number of hours per week spent watching television, videos, and movies, using a computer, or playing video games on television or on a computer. A simple questionnaire was administered to adolescents aged 12 to 14 years, based on a previously validated Youth Activity Questionnaire (Hernández et al., 2000). For adolescents aged 15 to 19 years, we used the International Physical Activity Questionnaire (IPAQ Short; Medina, Barquera, & Janssen, 2013), which included questions about normal screen time. Finally, screen time was classified as acceptable (up to 14 hr per week), borderline excessive (14–27 hr per week), and excessive (28 or more hours per week; Gutiérrez J.P., 2012; American Academy of Pediatrics Committee on Public Education, 2001; Morales‐Ruán, Hernández‐Prado, Gómez‐Acosta, Shamah‐Levy, & Cuevas‐Nasu, 2009).
2.3. Identification of plausible reporters
For this purpose, we used a specified method (Huang, Howarth, Lin, Roberts, & McCrory, 2004), in which cut‐off points of ±1 SD of the ratio of reported EI and the estimated energy requirement (EER) multiplied by 100 (EI/EER × 100) were considered (Huang et al., 2004; Rennie, Coward, & Jebb, 2007). EER was calculated using the U.S. Institute of Medicine formula for children and adolescents (9 to 18 years) and was expressed as a percentage of adequacy of EI. For adolescents >19 years, the formula for adults was used. Additionally, for adolescents with OW‐O, the equation of EER for weight maintenance in children and adolescents aged 3 to 18 years was used (based on the >19 years weight maintenance formula; National Research Council, 2005). We assume low levels of physical activity for whole sample, considering previous estimations from Mexican population, in order to avoid overestimating the energy requirements (Olaiz‐Fernández et al., 2006; Medina et al., 2013). The previous method used to identify plausible intake includes the coefficient of variation of EI (CVEI; that is, “the intraindividual variation of energy intake reported over the number of days of intake”; Aburto et al., 2015¸ Huang et al., 2004), the error of the equations for EER (CVEER), and the biological variation for total energy expenditure. Because we used an FFQ as a dietary assessment method, CVEI could not be calculated; however, we used the previous estimated values defined by Huang et al. (2004) for youth aged 9 to 13 years and 14 to 19 years. The ±1 SD cut‐off points for EI as a percentage of EER (EI/EER) were between ±19% and 23%, using the previous estimations (by sex and age range) proposed by Huang et al. (2004). Thus, plausibility range (% EI/EER) was between 81 to 119 in boys and 80 to 120 in girls of 12 to 13 years and between 80 to 120 in boys and 77 to 123 in girls of 14 to 19 years. Under‐ and over‐reporters were classified using EI/EER <1 SD and >1 SD, respectively.
2.4. Statistical analyses
Sex (male/female), age (12–13 years/14–15 years/16–17 years/18–19 years), area (urban/rural), SES (low/medium/high), and screen time (acceptable/borderline excessive/excessive) were expressed as categorical variables and energy, fibre, food weight, and SSB calories as continuous variables. The sociodemographic characteristics and BMI classification were compared using descriptive statistics and the chi‐square test. Additionally, characteristics of plausible and implausible reporters were also compared using the same test. Prevalence of OW‐O was calculated. Mean EI, macronutrients, and fibre were compared among individuals with OW‐O and normal weight using the Student's t test.
BMI‐for‐age z‐scores and OW‐O were considered dependent variables, whereas DED was defined as independent variable. On the other hand, DED was classified into low‐ (T1), medium‐ (T2), and high‐ (T3) density tertiles. To determine the association between DED and BMI‐for‐age z‐scores and OW‐O (confined to plausible reporters), we constructed multiple linear regression models and logistic regression models, respectively (this last analysis was included to observe the association, specifically with OW‐O and not only with BMI‐age z‐score). We developed bivariate and multivariate statistical models controlling for sociodemographic and dietary variables as well as for screen‐time data. Sex, age (continuous), area, region, fibre, screen time, and calories from SSBs were included as covariates, considering that these variables modified the association between DED and OW‐O (BMI‐age z‐score) or were related with the characteristics of the diet, in this population.
Potential collinearity was tested according to variance inflation factors, with results equalling <2. A level of P < 0.05 was considered significant. The 14.0 version of the Stata statistical program was used, employing the SVY module for complex samples, to consider the survey design.
2.5. Ethical considerations
All procedures involving human subjects were approved by the Ethics Committee and the Research and Biosafety Commissions of the National Institute of Public Health in Mexico. Written informed consent and informed assent were obtained from all subjects to participate and provide information.
3. RESULTS
3.1. Sample characteristics
Table 1 shows the sociodemographic characteristics of the sample stratified by energy report (plausible and implausible reporters). Significant differences were found by sex, region, and BMI classification. The combined prevalence of OW‐O was 35.4% in whole sample and 27.8% in plausible sample. Differences by area (P = 0.008) and SES (P < 0.001) were found in the whole sample, with a higher prevalence in urban area (37.8%) and in areas of high SES (40.1%) compared with rural areas (29.3%) and those of low SES (27.2%; Table S1).
Table 1.
Sociodemographic characteristics of Mexican adolescents by energy report (ENSANUT 2012)
| Sociodemographic characteristics | Full samplea (n = 2,044) | Plausible sample (n = 749) | Implausible sample (n = 1,295) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | P b | |
| Sex | ||||||||||
| Male | 1,049 | 55.6 | [52.1, 59.0] | 337 | 50.3 | [44.2, 56.4] | 712 | 58.8 | [54.7, 62.8] | 0.023 |
| Female | 995 | 44.4 | [41.0, 47.9] | 412 | 49.7 | [43.6, 55.8] | 583 | 41.2 | [37.2, 45.3] | |
| Age | ||||||||||
| 12 to 13 years | 604 | 28.0 | [25.3, 31.0] | 212 | 26.0 | [21.3, 31.3] | 392 | 29.4 | [26.0, 33.1] | 0.435 |
| 14 to 15 years | 516 | 25.2 | [22.5, 28.2] | 174 | 23.7 | [19.4, 28.5] | 342 | 26.1 | [22.7, 29.7] | |
| 16 to 17 years | 498 | 25.8 | [22.9, 28.9] | 190 | 27.5 | [22.5, 33.1] | 308 | 24.7 | [21.2, 28.6] | |
| 18 to 19 years | 426 | 21.0 | [18.4, 23.8] | 173 | 22.8 | [18.2, 28.2] | 253 | 19.8 | [16.9, 23.0] | |
| Region | ||||||||||
| North | 506 | 18.7 | [17.3, 20.3] | 185 | 17.9 | [17.2, 20.3] | 321 | 19.2 | [17.2, 21.4] | 0.048 |
| Centre | 861 | 49.3 | [46.8, 51.8] | 324 | 53.9 | [46.8, 51.8] | 537 | 46.5 | [43.1, 49.8] | |
| South | 677 | 32.0 | [29.8, 34.1] | 240 | 28.2 | [24.3, 32.6] | 437 | 34.3 | [31.4, 37.4] | |
| Areac | ||||||||||
| Urban | 1,291 | 72.0 | [69.9, 74.0] | 469 | 73.8 | [69.9, 77.4] | 822 | 70.8 | [67.8, 73.6] | 0.265 |
| Rural | 753 | 28.0 | [26.0, 30.1] | 280 | 26.2 | [22.6, 30.1] | 473 | 29.2 | [26.4, 32.2] | |
| BMI/aged | ||||||||||
| Normal | 1,297 | 64.6 | [61.3, 67.7] | 513 | 72.2 | [67.1, 76.7] | 784 | 59.8 | [55.7, 63.8] | <0.001 |
| Overweight and obesity | 747 | 35.4 | [32.3, 38.7] | 236 | 27.8 | [23.3, 32.9] | 511 | 40.2 | [36.2, 44.3] | |
| Socio‐economic statuse | ||||||||||
| Low | 675 | 28.7 | [26.1, 31.4] | 241 | 23.9 | [20.3, 28.0] | 434 | 31.7 | [28.1, 35.5] | 0.054 |
| Medium | 686 | 32.6 | [29.5, 35.9] | 252 | 33.6 | [28.1, 36.1] | 434 | 32.0 | [28.1, 36.1] | |
| High | 683 | 38.7 | [35.4, 42.1] | 256 | 42.5 | [36.6, 48.6] | 427 | 36.3 | [32.4, 40.5] | |
| Screen timef | ||||||||||
| Acceptable | 640 | 33.4 | [30.2, 36.8] | 239 | 32.6 | [27.3, 38.4] | 401 | 33.9 | [30.0, 38.2] | 0.168 |
| Borderline excessive | 680 | 36.5 | [33.2, 39.9] | 241 | 33.4 | [28.2, 38.9] | 439 | 38.5 | [34.3, 42.8] | |
| Excessive | 530 | 30.1 | [26.9, 33.4] | 206 | 34.0 | [28.3, 40.3] | 324 | 27.6 | [23.7, 31.8] | |
Note. ENSANUT 2012: 2012 Mexican National Health and Nutrition Survey; CI: confidence interval; values: weighted percentages and 95% CIs; BMI, body mass index.
Sample: n = 2,044; expanded sample: n = 21,337,591.
P value for differences between plausible and implausible samples based on a chi‐square test: P < 0.05.
Urban ≥ 2,500 inhabitants; rural < 2,500 inhabitants.
Overweight or obese was defined as BMI 1 SD based on the WHO Reference 2007 (De Onis et al., 2007).
Household characteristics were considered through principal components factor analysis categorized in tertiles.
Sample: n = 1,850; expanded sample: n = 19,135,744; plausible sample: n = 686 implausible sample: n = 1,164; acceptable: <14 hr/week; borderline excessive: 14–27 hr/week; excessive: 28 hr/week.
In both samples (the complete and plausible reporters), differences were found only between categories of DED and region. In the whole sample, the highest DED was noted in men (36.2%) in the north compared with the south (52.3% vs. 26.2%, respectively) and in urban areas (35.2%), but it was not significant for sex and area (Table 2). On the other hand, it was observed that the proportion of adolescents in front of screen and the mean of “screen time” showed a trend to increase with DED tertiles, particularly in those who spent from 14 to 27 hr per week, although this result was not significant (Table 2).
Table 2.
DED distribution according to the sociodemographic characteristics of Mexican adolescents (ENSANUT 2012)
| Sociodemographic characteristics | DED tertiles | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low T1 | Medium T2 | High T3 | Low T1 | Medium T2 | High T3 | |||||||||
| Full samplea (n = 2,044) | Plausible sampleb (n = 749) | |||||||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | P a | % | 95% CI | % | 95% CI | % | 95% CI | P c | |
| Full sample | 33.4 | [30.3, 36.6] | 33.2 | [30.0, 36.6] | 33.4 | [30.4, 6.5] | 30.5 | [27.9, 39.7] | 36.1 | [30.2, 42.4] | 30.4 | [25.6, 35.6] | ||
| Sex | ||||||||||||||
| Male | 30.6 | [26.5, 34.5] | 33.3 | [29.0, 41.0] | 36.1 | [32.2, 40.5] | 0.075 | 31.4 | [23.7, 40.1] | 37.2 | [29.1, 46.2] | 31.4 | [24.7, 39.0] | 0.754 |
| Female | 37.0 | [32.4, 41.8] | 33.0 | [28.3, 38.1] | 30.0 | [25.7, 34.6] | 35.7 | [27.9, 44.3] | 35.0 | [26.7, 44.4] | 29.3 | [22.6, 37.0] | ||
| Region | ||||||||||||||
| North | 18.6 | [14.6, 23.4] | 29.1 | [24.4, 34.3] | 52.3 | [46.4, 58.2] | <0.001 | 14.4 | [9.2, 21.8] | 30.2 | [22.7, 38.9] | 55.4 | [45.7, 64.6] | <0.001 |
| Centre | 35.3 | [30.2, 40.7] | 34.0 | [28.6, 39.8] | 30.7 | [26.0, 36.0] | 37.1 | [27.9, 47.4] | 38.2 | [28.7, 48.7] | 24.6 | [17.8, 32.9] | ||
| South | 39.2 | [34.4, 44.3] | 34.5 | [29.8, 39.4] | 26.3 | [22.1, 31.0] | 38.6 | [30.2, 47.7] | 35.9 | [28.3, 44.3] | 25.5 | [19.2, 33.2] | ||
| Areac | ||||||||||||||
| Urban | 32.6 | [28.7, 36.7] | 32.2 | [28.1, 36.5] | 35.2 | [31.4, 39.1] | 0.117 | 34.8 | [27.5, 42.8] | 37.1 | [30.0, 45.2] | 28.1 | [22.4, 34.6] | 0.245 |
| Rural | 35.4 | [30.9, 40.1] | 35.9 | [31.4, 40.6] | 28.7 | [24.4, 33.5] | 29.9 | [23.3, 37.4] | 33.4 | [26.4, 41.3] | 36.7 | [26.3, 41.3] | ||
| Socio‐economic statusd | ||||||||||||||
| Low | 32.0 | [27.6, 36.8] | 35.7 | [30.6, 41.2] | 32.3 | [27.3, 38.0] | 0.481 | 24.7 | [18.3, 32.5] | 36.3 | [28.2, 45.3] | 39.0 | [30.6, 48.1] | 0.123 |
| Medium | 37.3 | [31.5, 43.5] | 30.2 | [25.2, 35.7] | 32.5 | [26.8, 38.7] | 40.9 | [30.2, 52.6] | 30.4 | [21.9, 40.5] | 28.7 | [19.7, 39.6] | ||
| High | 31.2 | [26.1, 36.9] | 33.9 | [28.0, 40.3] | 34.9 | [30.4, 36.5] | 32.6 | [23.5, 43.1] | 40.6 | [30.5, 51.6] | 26.8 | [20.3, 34.6] | ||
| IMC/agee | ||||||||||||||
| Normal | 32.0 | [28.2, 36.1] | 34.4 | [40.3, 38.7] | 33.6 | [31.0, 38.6] | 0.421 | 32.0 | [25.0, 40.0] | 37.4 | [30.0, 45.4] | 30.6 | [30.0, 45.5] | 0.610 |
| Overweight and obesity | 36.0 | [30.9, 41.0] | 31.0 | [26.2, 36.1] | 33.0 | [28.0, 38.3] | 37.4 | [30.9, 41.0] | 32.8 | [24.4, 42.5] | 29.8 | [22.7, 37.9] | ||
| Screen timef (hr/week) | 20.8 SD 12.7 | 21.0 SD 13.5 | 22.7 SD 14.6 | 22.7 SD 14.8 | 18.8 SD 11.1 | 24.8 SD 19.6 | ||||||||
| Acceptable | 35.6 | [30.2, 41.5] | 35.1 | [29.1, 41.6] | 29.3 | [24.6, 34.6] | 0.458 | 33.8 | [24.8, 44.0] | 38.2 | [28.5, 49.6] | 28.0 | [20.5, 36.7] | 0.594 |
| Borderline excessive | 31.3 | [26.5, 36.5] | 32.2 | [27.3, 37.6] | 36.5 | [31.5, 42.1] | 30.4 | [22.3, 39.9] | 39.4 | [29.9, 49.7] | 30.2 | [29.9, 49.7] | ||
| Excessive | 33.6 | [27.1, 40.9] | 31.4 | [25.4, 38.1] | 35.0 | [28.7, 41.8] | 39.1 | [28.3, 451.1] | 29.6 | [20.7, 40.4] | 31.3 | [21.8, 42.6] | ||
Note. DED: dietary energy density; ENSANUT 2012: 2012 Mexican National Health and Nutrition Survey; CI: confidence interval; Values: weighted percentages and 95% CIs.
Sample: n = 2,044; expanded sample: n = 21,337,591.
Plausible sample: n = 749.
P values for differences between DED tertiles (Low T1, medium T2, high T3) based on a chi‐square test: P < 0.05.
Urban ≥ 2,500 inhabitants; rural ≤ 2,500 inhabitants.
Household characteristics were considered based on a principal components factor analysis categorized in tertiles.
Overweight or obese was defined as BMI 1 SD based on the WHO Reference 2007 (De Onis et al., 2007).
Sample: n = 1,850; expanded sample (n = 19,135,744); plausible sample: n = 686; implausible sample: n = 1,164; acceptable: <14 hr/week; borderline excessive: 14–27 hr/week; excessive: 28 hr/week.
3.2. Prevalence of plausible and implausible reporters
The prevalence of plausible reporters was 38.5%, more than 45% of adolescents being under‐reporters and approximately 14% over‐reporters. The prevalence of under‐reporters was higher in males as compared with females (53.8% vs. 39.5%), in the south compared with the north (53.0% vs. 44.8%), in adolescents >18 years (49.6%) compared with those aged 12 to 13 years (45.4%), and in OW‐O (62.7%) relative to normal weight subjects (39.1%; Table S2 and Figure 1). In contrast, the percentage of over‐reporters was higher in females (17.6%), in the north (18.4%), in adolescents aged 12 to 13 years (18.9%), and in individuals with a normal BMI‐for‐age (17.9%; Table S2 and Figure 1).
Figure 1.
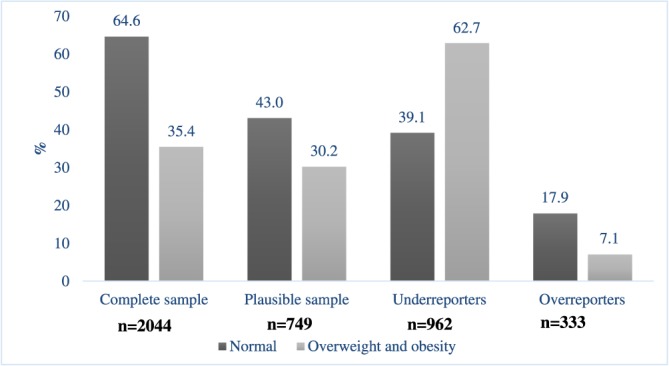
Prevalence of overweight and obesity in Mexican adolescents by energy report, ENSANUT 2012. Plausible sample % EI/EER = 19–23%, under‐reporters % EI/EER < 1 SD (<19%), over‐reporters % EI/EER > 1 SD (>23%)
3.3. DED, food (gr), and EI by BMI classification
Mean DED of the full sample was 177.8 ± 36.8 kcal/100 g and 177.0 ± 34.0 kcal/100 g for plausible reporters. Total food intake was higher in the full sample of adolescents with OW‐O compared with those in the normal BMI group (P = 0.027); however, the difference in total EI between normal weight and OW‐O was not statistically significant (Table 3). Mean EI from SSBs was 253.7 ± 216.4 kcal/day and slightly higher for adolescents with OW‐O than normal‐weight subjects. It was significant in plausible sample (347.1 kcal/day vs. 273.5 kcal/day; P = 0.005) but not in the sample as a whole. Overall, beverages represented 18.4% of total daily EI, with 12.8% pertaining to SSBs. Differences were found between normal and OW‐O plausible reporters as regards energy from foods that are not beverages and food that are not beverages intake, as well as in fibre, beverages energy, total energy, and total food intake. These were higher in adolescents with OW‐O compared with normal‐weight individuals (P ≤ 0.05).
Table 3.
DED as well as food (gr) and energy intake in Mexican adolescents by BMI classification (ENSANUT 2012)
| Variables | Full samplea | Normal | Overweight and obesityb | P c | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| DED (kcal/100 g) | 177.8 | 36.8 | 179.7 | 36.6 | 174.5 | 36.9 | 0.052 |
| Energy from foods that are not beverages (kcal/day) | 1,597.8 | 635.5 | 1,611.7 | 621.5 | 1,572.4 | 660.5 | 0.336 |
| Foods that are not beverages (g/day) | 1,006.0 | 433.0 | 1,004.45 | 422.4 | 1,008.6 | 452.3 | 0.889 |
| SSB energy (kcal/day) | 253.7 | 216.4 | 252.5 | 210.8 | 255.8 | 226.7 | 0.816 |
| Beverages energy (kcal/day) | 365.2 | 258.7 | 369.7 | 258.7 | 357.2 | 258.4 | 0.473 |
| Fibre (g/day) | 25.0 | 8.9 | 23.5 | 11.6 | 23.6 | 11.5 | 0.913 |
| Total energy (kcal/day) | 1,974.8 | 731.7 | 1,981.2 | 724.7 | 1,929.6 | 770.6 | 0.282 |
| Total food intake (g/day) | 2,656.7 | 1,031.7 | 2,602.0 | 1,025.7 | 2,756.5 | 1,034.6 | 0.027 |
| Plausible sample d | |||||||
| DED (kcal/100 g) | 177.0 | 34.0 | 178.2 | 33.8 | 173.7 | 34.1 | 0.220 |
| Energy from foods that are not beverages (kcal/day) | 1,802.3 | 402.8 | 1,734.0 | 356.9 | 1,971.4 | 470.3 | <0.001 |
| Foods that are not beverages (g/day) | 1,143.3 | 339.3 | 1,090.8 | 300.4 | 1,213.6 | 399.7 | <0.001 |
| SSB energy (kcal/day) | 293.9 | 205.9 | 273.5 | 178.3 | 347.1. | 263.8 | 0.005 |
| Beverages energy (kcal/day) | 419.5 | 238.3 | 404.3 | 208.1 | 466.7 | 305.6 | 0.042 |
| Fibre (g/day) | 26.4 | 9.6 | 25.0 | 8.9 | 30.1 | 10.6 | <0.001 |
| Total energy (kcal/day) | 2,221.8 | 437.7 | 2,138.3 | 368.7 | 2,438.1 | 533.9 | <0.001 |
| Total food intake (g/day) | 2,848.2 | 879.7 | 2,750.0 | 792.1 | 3,102.4 | 1,051.3 | <0.001 |
Note. DED: dietary energy density; ENSANUT 2012: 2012 Mexican National Health and Nutrition Survey; SD: standard deviation, SSB: sugar‐sweetened beverages. Values are means (95% CIs); weights were applied in all statistical procedures.
Full sample: n = 2,044; expanded sample n = 21,337,591.
Overweight or obese was defined as BMI of 1 SD based on the WHO Reference 2007 (De Onis et al., 2007).
P values for differences between normal and overweight and obesity samples were based on a t test P < 0.05.
Plausible sample: n = 749.
3.4. Association of DED with BMI‐for‐age z‐score and OW‐O
Tables 4 and 5 show the models used to determine the association of DED with the BMI‐for‐age z‐score and OW‐O (only for adolescents with plausible EI). A positive association between high DED (T3) and the BMI‐for‐age z‐score (β = 0.347, 95% CI [0.101, 0.594]; P = 0.006) was observed after controlling for potential confounders (model adjusted for sociodemographic variables, energy from SSBs and fibre). This indicates that the BMI‐for‐age z‐score was 0.347 units higher in adolescents with high DED (T3) relative to adolescents with low DED (T1; Model 2, Table 4). In Model 4, coefficients indicated that for each unit increase in DED, the BMI‐for‐age z‐score increased by 0.003 kg/m2 on average (95% CI [0.001, 0.006]; P = 0.030).
Table 4.
Association between DED and BMI‐for‐age z‐score in Mexican adolescents with plausible reports of energy (ENSANUT 2012)
| DED (n = 749) | Predictive values | Model values | |||
|---|---|---|---|---|---|
| Coefficient β | 95% CI | P | R 2 | P | |
| Model 1a | |||||
| Low DED (T1) | |||||
| Medium DED (T2) | −0.161 | [−0.507, 0.185] | 0.360 | 0.003 | 0.638 |
| High DED (T3) | −0.089 | [−0.346, 0.169] | 0.499 | ||
| Model 2 adjustedb | |||||
| Low DED (T1) | |||||
| Medium DED (T2) | 0.045 | [−0.291, 0.300] | 0.932 | 0.183 | <0.001 |
| High DED (T3) | 0.347 | [0.101, 0.594] | 0.006 | ||
| Model 3c | |||||
| Continuous DED | −0.002 | [−0.005, 0.001] | 0.157 | 0.004 | 0.157 |
| Model 4 adjustedd | |||||
| DED adjusted | 0.003 | [0.001, 0.006] | 0.030 | 0.175 | <0.001 |
Note. DED: Dietary energy density; ENSANUT 2012: 2012 Mexican National Health and Nutrition Survey; CI: confidence interval; BMI, body mass index. Coefficients were obtained through linear regression models using the low DED tertile as reference. The BMI‐for‐age z‐score was used as a continuous variable. Models were restricted to plausible reporters (n = 749). Survey weights were applied in all statistical procedures.
Bivariate.
Adjusted by age, sex, region, rural or urban residence, socio‐economic status, energy from sugar‐sweetened beverages, and fibre.
Continuous DED.
Continuous DED adjusted by age, sex, region, rural or urban residence, socio‐economic status, energy from sugar‐sweetened beverages, and fibre.
Table 5.
Association between DED and OW‐O in Mexican adolescents with plausible reports of energy (ENSANUT 2012)
| DED (n = 749) | OR | CI 95% | P |
|---|---|---|---|
| Model 1a | |||
| Low DED (T1) | |||
| Medium DED (T2) | 0.748 | [0.395, 1.419] | 0.374 |
| High DED (T3) | 0.830 | [0.464, 1.484] | 0.529 |
| Model 2 Adjustedb | |||
| Low DED (T1) | |||
| Medium DED (T2) | 0.965 | [0.498, 1.868] | 0.885 |
| High DED (T3) | 1.829 | [0.498, 1.868] | 0.068 |
| Model 3c | |||
| Continuous DED | 0.996 | [0.989, 1.002] | 0.224 |
| Model 4 Adjustedd | |||
| DED adjusted | 1.005 | [0.988, 1.013] | 0.150 |
Note. DED: Dietary energy density; ENSANUT 2012: 2012 Mexican National Health and Nutrition Survey; CI: confidence interval; OR: odds ratio. ORs were obtained through logistic regression models using overweight or obese dichotomously and the low DED tertiles as reference. Overweight or obese was defined as BMI‐for‐age > 1 SD based on the WHO Reference 2007 (22). Models were restricted to plausible reporters (n = 749).
Bivariate.
Adjusted by age, sex, region, rural or urban residence, socio‐economic status, energy from sugar‐sweetened beverages and fibre.
Continuous DED.
Adjusted by age, sex, region, rural or urban residence, socio‐economic status, energy from sugar‐sweetened beverages, and fibre.
The odds of being OW‐O were 1.83 (95% CI [0.95, 3.50]; P = 0.068) higher in plausible reporters adolescents in the high as compared with the low DED categories, but the association was not statistically significant (Table 5, Model 2). Models were also adjusted for the same covariates and screen time, but no changes were found (data not shown). On the other hand, the models covering the full sample (including under‐ and over‐reporters) showed inverse associations among DED, OW‐O, and BMI‐for‐age z‐scores in Mexican adolescents, except for the models adjusted by energy under‐reporting (Tables S3 and S4).
4. DISCUSSION
The present study analysed the association of DED with OW‐O and the BMI‐for‐age z‐scores in Mexican adolescents from the ENSANUT 2012. The most important finding was a positive association between high DED and BMI‐for‐age z‐score in adolescent plausible energy reporters, but no significant association with OW‐O. Our results are in agreement with a number of cross‐sectional studies that have established a positive relationship between DED and BMI (z‐score) in children (Aburto et al., 2015) and adults (Hartline, Rose, Johnson, Rise, & Webber, 2009; Ledikwe et al., 2006; Mendoza et al., 2007). They are also in line with WHO recommendations (WHO, 2003) based on findings that high‐DED diets contribute to excess body weight. Results for adolescents, however, remain inconclusive. Several studies have found no association between DED and BMI (z‐score) in adolescents (Kring & Heitmann, 2008; Murakami et al., 2012a; Van Sluijs et al., 2016; Zhou et al., 2015), whereas others have reported positive associations with fat mass (Johnson et al., 2009b), fat mass index (Ambrosini et al., 2012; McCaffrey et al., 2008), waist circumference, and other weight‐related measurements (Schroder et al., 2013).
Our findings concerning the association between DED and OW‐O in plausible and full‐sample adolescents were similar to those reported by Murakami et al. (2012a) and O'Sullivan et al. (2015). Nevertheless, given methodological differences among studies in the DED calculation method, dietary assessment tool, energy misreporting estimates, and other operational factors, results should not be compared.
According to WCRF, the mean DED reported in our study (177 kcal/100 g) was above of the recommendations of WCRF (125 kcal/100 g; WCRF/AICR, 2007). Various studies of adolescents in Japan (Murakami et al., 2012a), Germany (Alexy et al., 2004), and Denmark (Kring & Heitmann, 2008) have reported a lower mean DED than that observed in our study and have found no association with OW‐O. In contrast, countries such as England (Johnson et al., 2009b) and Ireland (McCaffrey et al., 2008) have reported a higher mean DED and have found a positive association with weight gain in adolescents. Similarly, in Mexico, a positive association has been observed between DED (175 kcal/100 g) and OW‐O among children (Aburto et al., 2015). In this regard, it should be noted that our findings reflected a slightly higher DED in the full sample of adolescents than in children; although in adolescents with OW‐O, the mean DED was 5.3 kcal/100 g lower than in those with normal weight.
The lack of association between DED and OW‐O in plausible reporters can be explained by the reduction of the sample size when implausible reporters were excluded from analysis. It may also relate to the loss of variability when the BMI‐for‐age z‐score was categorized because significant results were observed when the latter was included in the model as the outcome variable. Conversely, in the whole sample, said lack of association could be the result of under‐reported EI on the part of adolescents with OW‐O and also because EI was higher in normal weighted adolescents compared with those with OW‐O.
Energy under‐reporting is commonly seen in individuals with OW‐O (Collins, Watson, & Burrows, 2010; Heitmann & Lissner, 1995; Lioret et al., 2011), which is in line with our findings, where more than 60% of the under‐reporters had excess body weight. This could reflect a deliberate omission in reported consumption of energy dense foods and an over‐reporting of consumption of healthy foods such as fruits and vegetables among adolescents with OW‐O. On the other hand, it is possible that adolescents with OW‐O identified as under‐reporters had changed their diet in an attempt to decrease their body weight. However, this information was not considered in our analysis; the results of which should thus be interpreted cautiously. It is important to consider that under‐reporting does not derive solely from changes in diet but is also related to under‐recording (Livingstone & Robson, 2000). This is especially frequent among adolescents due to irregularity in food consumption, the omission of meal times, and out‐of‐home eating patterns (Livingstone, Robson, & Wallace, 2004) including the consumption of HSFAS products, such as sweets or snacks, that are likely to be forgotten (Heitmann & Lissner, 1995; Lioret et al., 2011). In addition, recording dietary intake is often an annoying process for adolescents, which coupled with a lack of cooperation could increase the reporting error (Collins et al., 2010).
In line with the above, we found that the proportion of plausible reporters was 38.5%, lower than in previous studies that had also considered misreporting in their analysis of Mexican (61.2%; Aburto et al., 2015), Japanese (53.2%; Murakami, Miyake, Sasaki, Tanaka, & Arakawa, 2012b, American (72.3%; Murakami & Livingstone, 2016), and British (47%; Murakami, McCaffrey, & Livingstone, 2013) children and adolescents.
There are several limitations to our study. First, a cross‐sectional design made it impossible to establish a causal association between DED and BMI‐for‐age or OW‐O in adolescents. Second, the use of the FFQ methodology could be related with the high under‐reporting found in our study, considering that foods that are not listed in this cannot be reported, resulting in an underestimation of EI. However, the food list included in the questionnaire that we used contributes with approximately 90% of the energy in Mexican population (Ramírez‐Silva et al., 2016). Moreover, the FFQ did not allow identifying the food preparation method to some food in the list, which includes their water content, whereby FFQ would not be the best instrument to perform an accurate estimation of the DED. Other studies have reported a positive association between DED and OW‐O using a 24‐hr recall (Schroder et al., 2013) or dietary records (Johnson et al., 2009b; Ambrosini et al., 2012) but not an FFQ (O'Sullivan et al., 2015). Third, the method we used to identify plausible reporters was based on the equations of the U.S. Institute of Medicine for calculating the EER, which are body‐weight‐dependent formulae. Because our outcome variable was BMI, also a function of body weight, and both the exclusion criteria for primary exposure and the outcome were indirectly based on body weight, our approach to identifying plausible reporters could have led to a spurious association between DED and BMI resulting from selection bias. Our results must therefore be interpreted carefully. A fourth limitation refers to the fact that we did not have a specific and valid physical activity measurement for the entire population, so we used a low‐activity level to conservatively estimate the EER. Furthermore, we used “screen time” variable as a proxy measurement for a sedentary lifestyle, given evidence of its association with a high DED intake in adolescents (Phillips et al., 2004). Our study found no association between screen time and DED but observed an increase in screen‐time hours proportional to the increase in DED tertiles. Finally, the proportion of individuals with plausible energy reports was significantly lower than those excluded by under‐reporting (the majority with OW‐O), with a consequent decrease in the representativeness of the association between DED and OW‐O results for the general population of Mexican adolescents.
As for the strengths of the study, this is the first research in Mexico to provide information on the ways in which DED is associated with excess body weight in adolescents. Furthermore, data were drawn from a nationally representative survey, lending external validity to the study in extrapolating the descriptive results—not the association—to the entire population. We were thus able to establish that DED was higher in the north and in urban areas, which is consistent with previous evidence concerning the Mexican population (Aburto et al., 2016; López‐Olmedo et al., 2016) and with the high prevalence of OW‐O youth with those characteristics. Our results can therefore contribute to the design of diet‐related food and nutrition interventions targeted to Mexican adolescents. Another strength concerns the fact that our estimates and analyses were restricted to plausible reporters. This allowed for improving the association between DED and BMI‐for‐age z‐score despite the exclusion of a great number of individuals with excess body weight. The association between DED and BMI‐for‐age z‐score was also improved with the inclusion of energy from drinks (mainly SSBs) as a covariate in the models with regard to the high consumption of these beverages in Mexico (Barquera et al., 2008; Sánchez et al., 2016). Overall, our study provides useful evidence on the distribution of DED and OW‐O broken down by sociodemographic characteristics in Mexican adolescents.
It is recommended that future studies use a prospective analysis, consider different adjustment factors and variables employ alternative dietary assessment methods, and rely on other anthropometric indicators in addition to BMI. Our study provides evidence relevant to public health that will open the way to new research on diet in adolescents, emphasizing the role of DED as a risk factor for OW‐O and taking into consideration other determinants previously mentioned.
5. CONCLUSION
In summary, we found a positive association between high DED and the BMI‐for‐age z‐score in adolescents with plausible energy reports. This serves as a basis for issuing evidence‐based recommendations towards the development and implementation of nutritional strategies aimed at reducing the prevalence of OW‐O in the younger Mexican population. Our findings can offer significant support in the reduction of DED and in the promotion of healthy nutrition, especially in places characterized by a high prevalence of adolescents.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
All authors were responsible for the study conceptualization and design. AA and SR were responsible for the data analysis. All authors contributed to the interpretation of the results. The manuscript was drafted by AA with correction and critical revision from SR and TS. All authors read and approved the final manuscript.
Supporting information
Data S1. Supporting information
Arango‐Angarita A, Shamah‐Levy T, Rodríguez‐Ramírez S. Dietary energy density is associated with body mass index‐for‐age in Mexican adolescents. Matern Child Nutr. 2019;15:e12664 10.1111/mcn.12664
REFERENCES
- Aburto, T. C. , Cantoral, A. , Hernández, L. , Alicia, L. , Carriquiry, A. L. , & Rivera, J. A. (2015). Usual dietary energy density distribution is positively associated with excess body weight in Mexican children. The Journal of Nutrition, 145(7), 1524–1530. 10.3945/jn.114.206359 [DOI] [PubMed] [Google Scholar]
- Aburto, T. C. , Pedraza, L. S. , Sánchez, T. G. , Batis, C. , & Rivera, J. A. (2016). Discretionary foods have a high contribution and fruit, vegetables, and legumes have a low contribution to the total energy intake of the Mexican population. The Journal of Nutrition, 146(9), 1881S–1887S. 10.3945/jn.115.219121 [DOI] [PubMed] [Google Scholar]
- Alexy, U. , Sichert‐Hellert, W. , Kersting, M. , & Schultze‐Pawlitschko, V. (2004). Pattern of long‐term fat intake and BMI during childhood and adolescence results of the DONALD Study. International Journal of Obesity and Related Metabolic Disorders, 28(10), 1203–1209. [DOI] [PubMed] [Google Scholar]
- Ambrosini, G. L. , Emmett, P. M. , Northstone, K. , Howe, L. D. , Tilling, K. , & Jebb, S. A. (2012). Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. International Journal of Obesity, 36(10), 1299–1305. 10.1038/ijo.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Public Education . (2001). American Academy of Pediatrics: Children, adolescents, and television. Pediatrics, 107(2), 423–426. [DOI] [PubMed] [Google Scholar]
- Barquera, S. , Campos, I. , & Rivera, J. A. (2013). Mexico attempts to tackle obesity: The process, results, push backs and future challenges. Obesity Reviews, 14(S2), 69–78. 10.1111/obr.12096 [DOI] [PubMed] [Google Scholar]
- Barquera, S. , Hernández, L. , Tolentino, L. , Espinosa, J. , Ng, S. W. , Rivera, J. A. , & Popkin, B. M. (2008). Energy intake from beverages is increasing among Mexican adolescents and adults. The Journal of Nutrition, 138(12), 2454–2461. 10.3945/jn.108.092163 [DOI] [PubMed] [Google Scholar]
- Birch, L. L. , & Deysher, M. (1986). Caloric compensation and sensory specific satiety: Evidence for self‐regulation of food intake by young children. Appetite, 7(4), 323–331. [DOI] [PubMed] [Google Scholar]
- Collins, C. , Watson, J. , & Burrows, T. (2010). Measuring dietary intake in children and adolescence in the context of overweight and obesity. International Journal of Obesity (London), 34(7), 103–1115. 10.1038/ijo.2009.241 [DOI] [PubMed] [Google Scholar]
- De Onis, M. , Onyango, A. W. , Borghi, E. , Siyam, A. , Nishida, C. , & Siekmann, J. (2007). Development of a WHO growth reference for school‐aged children and adolescents. Bulletin of the World Health Organization, 85(9), 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denova‐Gutiérrez, E. , Ramírez‐Silva, I. , Rodríguez‐Ramírez, S. , Jiménez‐Aguilar, A. , Shamah‐Levy, T. , & Rivera‐Dommarco, J. A. (2016). Validity of a Food Frequency Questionnaire to assess food intake in Mexican adolescent and adult population. Salud Pública de México, 58(6), 617–628. 10.21149/spm.v58i6.7862 [DOI] [PubMed] [Google Scholar]
- Du, H. , van der An, D. L. , Ginder, V. , Jebb, S. A. , Forouhi, N. G. , Wareham, N. J. , … Feskens, E. J. (2009). Dietary energy density in relation to subsequent changes of weight and waist circumference in European men and women. PLoS One, 4(4), e5339 10.1371/journal.pone.0005339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Dantés, H. , Fullman, N. , Lamadrid‐Figueroa, H. , Cahuana‐Hurtado, L. , Darney, B. , Ávila‐Burgos, L. , … Lozano, R. (2016). Dissonant health transition in the states of Mexico, 1990–2013: A systematic analysis for the Global Burden of Disease Study. The Lancet, 388(10058), 2386–2402. 10.1016/S0140-6736(16)31773-1 [DOI] [PubMed] [Google Scholar]
- Gurnani, M. , Birken, C. , & Hamilton, J. (2015). Childhood Obesity causes, consequences, and management. Pediatric Clinics of North America, 62(4), 821–840. 10.1016/j.pcl.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Gutiérrez, J. P. (2013). Clasificación socioeconómica de los hogares en la ENSANUT 2012 [Household socioeconomic classification in the National Health and Nutrition Survey 2012]. Salud Pública de México, 55(S2), S341–S346. [PubMed] [Google Scholar]
- Gutiérrez J. P., Rivera‐Dommarco J., Shamah‐Levy T., Villalpando‐Hernández S., Franco A., Cuevas‐Nasu L., … Hernández‐Ávila M. (Eds.) (2012). Encuesta nacional de salud y nutrición 2012: resultados nacionales, Primera edición [National Health and Nutrition Survey 2012: National results, first edition] ed. Instituto Nacional de Salud Pública (MX): Secretaría de Salud, Cuernavaca, Morelos, México. [Google Scholar]
- Habitch, J. P. (1974). Estandarización de métodos epidemiológicos cuantitativos sobre el terreno [Standardization of quantitative epidemiological methods in the field]. Boletín de la Oficina Sanitaria Panamericana, 76(5), 375–384. [PubMed] [Google Scholar]
- Hartline, H. , Rose, D. , Johnson, C. C. , Rise, J. C. , & Webber, L. S. (2009). Energy density of foods, but not beverages, is positively associated with body mass index in adult women. European Journal of Clinical Nutrition, 63(12), 1411–1418. 10.1038/ejcn.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann, B. L. , & Lissner, L. (1995). Dietary underreporting by obese individuals—Is it specific or non‐specific? BMJ, 311(7011), 986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández, B. , Gortmarker, S. L. , Larid, N. M. , Colditz, G. , Parra‐Cabrera, S. , & Peterson, K. E. (2000). Validez y reproducibilidad de un cuestionario de actividad e inactividad física para escolares de la ciudad de México [Validity and reproducibility of a questionnaire on physical activity and non‐activity for school children in Mexico City]. Salud Pública de México, 42(4), 315–323. [PubMed] [Google Scholar]
- Hernández‐Cordero, S. , Cuevas‐Nasu, L. , Morán‐Ruán, M. C. , Méndez‐Gómez Humarán, I. , & Ávila‐Arcos, M. (2017). Overweight and obesity in Mexican children and adolescents during the last 25 years. Nutrition & Diabetes, 13(7), e247 10.1038/nutd.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. T. , Howarth, N. C. , Lin, B. H. , Roberts, S. B. , & McCrory, M. A. (2004). Energy intake and meal portions: Associations with BMI percentile in U.S. children. Obesity Research, 12(11), 1875–1885. 10.1038/oby.2004.233 [DOI] [PubMed] [Google Scholar]
- Johnson, L. , Mander, A. P. , Jones, L. R. , Emmett, P. M. , & Jebb, S. A. (2008a). A prospective analysis of dietary energy density at age 5 and 7 years and fatness at 9 years among UK children. International Journal of Obesity, 32(4), 586–593. 10.1038/sj.ijo.0803746 [DOI] [PubMed] [Google Scholar]
- Johnson, L. , Mander, A. P. , Jones, L. R. , Emmett, P. M. , & Jebb, S. A. (2008b). Energy‐dense, low‐fiber, high‐fat dietary pattern is associated with increased fatness in childhood. The American Journal of Clinical Nutrition, 87(4), 846–854. [DOI] [PubMed] [Google Scholar]
- Johnson, L. , Wilks, D. C. , Lindroos, A. K. , & Jebb, S. A. (2009a). Reflections from a systematic review of dietary energy density and weight gain: Is the inclusion of drinks valid? Obesity Reviews, 10(6), 681–692. 10.1111/j.1467-789X.2009.00580.x [DOI] [PubMed] [Google Scholar]
- Johnson, L. , Van Jaarsveld, C. H. , Emmett, P. M. , Rogers, I. S. , Ness, A. R. , Hattersley, A. T. , … Jebb, S. A. (2009b). Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS One, 4(3), e4594 10.1371/journal.pone.0004594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring, S. I. , & Heitmann, B. L. (2008). Fiber intake, not dietary energy density, is associated with subsequent change in BMI z‐score among sub‐groups of children. Obesity Facts, 1(6), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy, K. E. , Birch, L. L. , & Rolls, B. J. (2008). Reducing the energy density of multiple meals decreases the energy intake of preschool‐age children. The American Journal of Clinical Nutrition, 88(6), 1459–1468. [DOI] [PubMed] [Google Scholar]
- Ledikwe, J. H. , Blanck, H. M. , Kettel, L. , Serdula, M. K. , Seymour, J. D. , Tohill, B. , & Rolls, B. (2006). Dietary energy density is associated with energy intake and weight status in US adults. The American Journal of Clinical Nutrition, 83(6), 1362–1368. [DOI] [PubMed] [Google Scholar]
- Lioret, S. , Touvier, M. , Balin, M. , Huybrechts, I. , Dubuisson, C. , Dufour, A. , … Lafay, L. (2011). Characteristics of energy under‐reporting in children and adolescents. The British Journal of Nutrition, 105(11), 1671–1680. 10.1017/S0007114510005465 [DOI] [PubMed] [Google Scholar]
- Livingstone, M. B. , & Robson, P. J. (2000). Measurement of dietary intake in children. The Proceedings of the Nutrition Society, 59(2), 279–293. [DOI] [PubMed] [Google Scholar]
- Livingstone, M. B. , Robson, P. J. , & Wallace, J. M. (2004). Issues in dietary intake assessment of children and adolescents. The British Journal of Nutrition, 92(S2), S213–S222. [DOI] [PubMed] [Google Scholar]
- López‐Olmedo, C. A. , Rodríguez‐Ramírez, S. , Ramírez‐Silva, I. , Espinosa‐Montero, J. , Hernández‐Barrera, L. , … Rivera, J. A. (2016). Usual intake of added sugars and saturated fats is high while dietary fiber is low in the Mexican population. The Journal of Nutrition, 146(9), 1856S–1865S. 10.3945/jn.115.218214 [DOI] [PubMed] [Google Scholar]
- McCaffrey, T. A. , Rennie, K. L. , Kerr, M. A. , Wallace, J. M. , Hannon‐Fletcher, M. , Coward, W. A. , … Livingstone, B. (2008). Energy density of the diet and change in body fatness from childhood to adolescence: Is there a relation? The American Journal of Clinical Nutrition, 87(5), 1230–1237. [DOI] [PubMed] [Google Scholar]
- Medina, C. , Barquera, S. , & Janssen, I. (2013). Validity and reliability of the International Physical Activity Questionnaire among adults in Mexico. Revista Panamericana de Salud Pública, 34(1), 21–28. [PubMed] [Google Scholar]
- Mendoza, J. A. , Drewnowski, A. , & Christakis, D. A. (2007). Dietary energy density is associated with obesity and the metabolic syndrome in US adults. Diabetes Care, 30(4), 974. [DOI] [PubMed] [Google Scholar]
- Morales‐Ruán, M. , Hernández‐Prado, B. , Gómez‐Acosta, L. , Shamah‐Levy, T. , & Cuevas‐Nasu, L. (2009). Obesity, overweight, screen time and physical activity in Mexican adolescents. Salud Pública de México, 51(S4), S613–S620. [DOI] [PubMed] [Google Scholar]
- Murakami, K. , & Livingstone, M. B. (2016). Prevalence and characteristics of misreporting of energy intake in US children and adolescents: National Health and Nutrition Examination Survey (NHANES) 2003–2012. British Journal of Nutrition, 115(2), 294–304. 10.1017/S0007114515004304 [DOI] [PubMed] [Google Scholar]
- Murakami, K. , McCaffrey, T. A. , & Livingstone, M. B. E. (2013). Dietary glycemic index and glycemic load in relation to food and nutrient intake and indices of body fatness in British children and adolescents. The British Journal of Nutrition, 110(8), 1512–1523. 10.1017/S000711451300072X [DOI] [PubMed] [Google Scholar]
- Murakami, K. , Miyake, Y. , Sasaki, S. , Tanaka, K. , & Arakawa. (2012a). An energy‐dense diet is cross‐sectionally associated with an increased risk of overweight in male children, but not in female children, male adolescents, or female adolescents in Japan: The Ryukyus Child Health Study. Nutrition Research, 32(7), 486–449. 10.1038/sj.ijo.0802708 [DOI] [PubMed] [Google Scholar]
- Murakami, K. , Miyake, Y. , Sasaki, S. , Tanaka, K. , & Arakawa, M. (2012b). Characteristics of under‐ and over‐reporters of energy intake among Japanese children and adolescents: The Ryukyus Child Health Study. Nutrition, 28(5), 532–538. 10.1016/j.nut.2011.08.011 [DOI] [PubMed] [Google Scholar]
- National Research Council (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington (DC): The National Academies Press. [Google Scholar]
- O'Connor, L. , Walton, J. , & Flynn, A. (2013). Dietary energy density and its association with the nutritional quality of the diet of children and teenagers. Journal of Nutritional Science, 15(2), e10 10.1017/jns.2013.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaiz‐Fernández, G. , Rivera‐Dommarco, J. , Shamah‐Levy, T. , Rojas, R. , Villalpando‐Hernández, S. , Hernández‐Ávila, M. , & Sepúlveda‐Amor, J. (2006). Encuesta Nacional de Salud y Nutrición 2006 [National Health and Nutrition Survey]. (pp. 1–132). Instituto Nacional de Salud Pública: 2006. Cited Dec Cuernavaca Morelos (México).
- O'Sullivan, T. A. , Bremner, A. P. , Bremer, H. K. , Seares, M. E. , Beilin, L. J. , Mori, T. A. , … Oddy, W. H. (2015). Dairy product consumption, dietary nutrient and energy density and associations with obesity in Australian adolescents. Journal of Human Nutrition and Dietetics, 28(5), 452–464. 10.1111/jhn.12264 [DOI] [PubMed] [Google Scholar]
- Pérez‐Escamilla, R. , Obbagy, J. E. , Altman, J. M. , Essery, E. V. , McGrane, M. M. , Wong, Y. P. , … Williams, C. L. (2012). Dietary energy density and body weight in adults and children: A systematic review. Journal of the Academy of Nutrition and Dietetics, 112(5), 671–684. 10.1016/j.jand.2012.01.020 [DOI] [PubMed] [Google Scholar]
- Phillips, S. M. , Bandini, L. G. , Naumova, E. N. , Cyr, H. , Colclough, S. , Dietz, W. H. , & Must, A. (2004). Energy‐dense snack food intake in adolescence: Longitudinal relationship to weight and fatness. Obesity Research, 12(3), 461–472. [DOI] [PubMed] [Google Scholar]
- Ramírez‐Silva, I. , Jiménez‐Aguilar, A. , Valenzuela‐Bravo, D. , Martínez‐Tapia, B. , Rodríguez‐Ramírez, S. , Gaona‐Pineda, E. B. , … Shamah‐Levy, T. (2016). Methodology for estimating dietary data from the semi‐quantitative food frequency questionnaire of the Mexican National Health and Nutrition Survey 2012. Salud Pública de México, 58(6), 629–638. 10.21149/spm.v58i6.7974 [DOI] [PubMed] [Google Scholar]
- Rennie, K. L. , Coward, A. , & Jebb, S. A. (2007). Estimating under‐reporting of energy intake in dietary surveys using an individualised method. The British Journal of Nutrition, 97(6), 1169–1176. [DOI] [PubMed] [Google Scholar]
- Rivera, J. A. , de Cossio, T. G. , Pedraza, L. S. , Aburto, T. C. , Sanchez, T. G. , & Martorell, R. (2014). Childhood and adolescent overweight and obesity in Latin America: A systematic review. The Lancet Diabetes and Endocrinology, 2(4), 321–332. 10.1016/S2213-8587(13)70173-6 [DOI] [PubMed] [Google Scholar]
- Rocchini, A. P. (2011). Childhood obesity and coronary heart disease. The New England Journal of Medicine, 365, 1927–1929. 10.1056/NEJMe1110898 [DOI] [PubMed] [Google Scholar]
- Rolls, B. (2009). The relationship between dietary energy density and energy intake. Physiol Behave, 97(5), 609–615. 10.1016/j.physbeh.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Martínez, M. , Shamah‐Levy, T. , Franco‐Núñez, A. , Villalpando, S. , Cuevas‐Nasu, L. , Gutiérrez, J. P. , & Rivera‐Dommarco, J. Á. (2013). Encuesta Nacional de Salud y Nutrición 2012: diseño y cobertura [National Health and Nutrition Survey 2012: Design and coverage]. Salud Pública de México, 55(S2), 332–340. [PubMed] [Google Scholar]
- Rouhani, M. H. , Haghighatdoost, F. , Surkan, P. J. , & Azadbakht, L. (2016). Associations between dietary energy density and obesity: A systematic review and meta‐analysis of observational studies. Nutrition, 32(10), 1037–1047. 10.1016/j.nut.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Sánchez, T. G. , Batis, C. , Lutter, C. K. , & Rivera, J. A. (2016). Sugar‐sweetened beverages are the main sources of added sugars intake in the Mexican population. The Journal of Nutrition, 146(S9), 1888S–1896S. 10.3945/jn.115.220301 [DOI] [PubMed] [Google Scholar]
- Schroder, H. , Mendez, M. A. , Gomez, S. F. , Fito, M. , Ribas, L. , Aranceta, J. , & Serra‐Majem, L. (2013). Energy density, diet quality, and central body fat in a nationwide survey of young Spaniards. Nutrition, 29(11–12), 1350–1355. 10.1016/j.nut.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Shamah‐Levy, T. , Villalpando‐Hernández, S. , & Rivera‐Dommarco, J. (2006). Manual de procedimientos para proyectos de nutrición. Cuernavaca, Morelos: Instituto Nacional de Salud Pública; Retrieved from: http://www.salud.gob.mx/unidades/cdi/documentos/proy_nutricion.pdf [Google Scholar]
- Van Sluijs, E. M. , Sharp, S. J. , Ambrosini, G. L. , Cassidy, A. , Griffin, S. J. , & Ekelund, U. (2016). The independent prospective associations of activity intensity and dietary energy density with adiposity in young adolescents. The British Journal of Nutrition, 14(115), 921–929. 10.1017/S0007114515005097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernarelli, J. A. , Mitchell, D. C. , Hartman, T. J. , & Rolls, B. J. (2011). Dietary energy density is associated with body weight status and vegetable intake in US children. The Journal of Nutrition, 141(12), 2204–2210. 10.3945/jn.111.146092.nomic [DOI] [PMC free article] [PubMed] [Google Scholar]
- WCRF/AICR . (2007). Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- WHO . (2003). Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation. Geneva: World Health Organization. [Google Scholar]
- Zhou, X. , Xue, H. , Duan, R. , Liu, Y. , Zhang, L. , Harvey, L. , & Cheng, G. (2015). The cross‐sectional association of energy intake and dietary energy density with body composition of children in Southwest China. Nutrients, 7(7), 5396–5412. 10.3390/nu7075228 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


