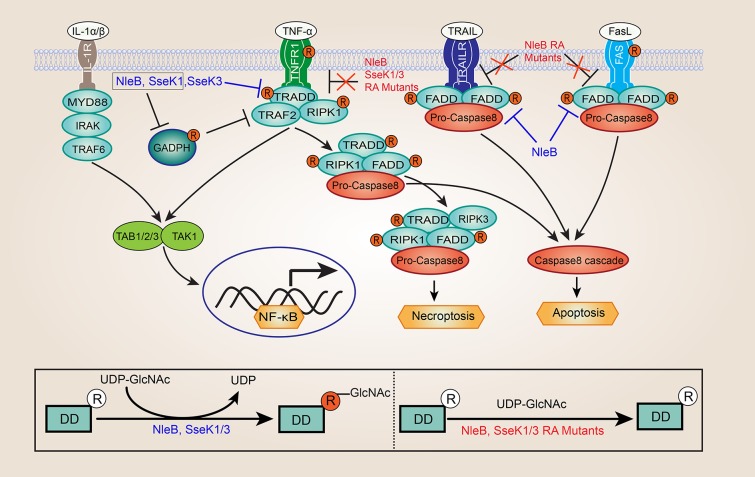
Figure 1.
Inhibition of NF-κB signaling and death receptor signaling by NleB and SseK1/3. GlcNAcylation of GAPDH by NleB/SseK1 would suppress TRAF2 polyubiquitination and NF-κB activation. GlcNAcylation of death domain (DD) proteins by NleB and SseK1/3 abrogates homotypic and heterotypic death receptors/adaptors interactions and the assembly of TNFR1 complex, leading to disrupting TNF signaling in EPEC or Citrobacter rodentium infected cells, including NF-κB signaling, apoptosis, and necroptosis. NleB also blocked Fas ligand and TNF-associated apoptosis-inducing ligand (TRAIL)-induced cell death by preventing assembly of the canonical death inducing signaling complex (DISC). In contrast, interleukin-1 receptor-associated kinase1 (IRAK1), and myeloid differentiation primary response 88 (MYD88), lacking the conserved arginine were not GlcNAcylated by NleB and SseK1/3. Besides, the site-directed RA mutants of NleB (NleBArg13/53/159/293Ala), SseK1 (SseK1Arg30/158/339Ala), and SseK3 (SseK3Arg153/184/305/335Ala) abolished or attenuated the capability of enzyme activity toward their death domain-containing targets during infection, and loss of self-GlcNAcylation of NleB, SseK1, and SseK3 couldn't inhibit TNFα- or TRAIL-induced cell death.