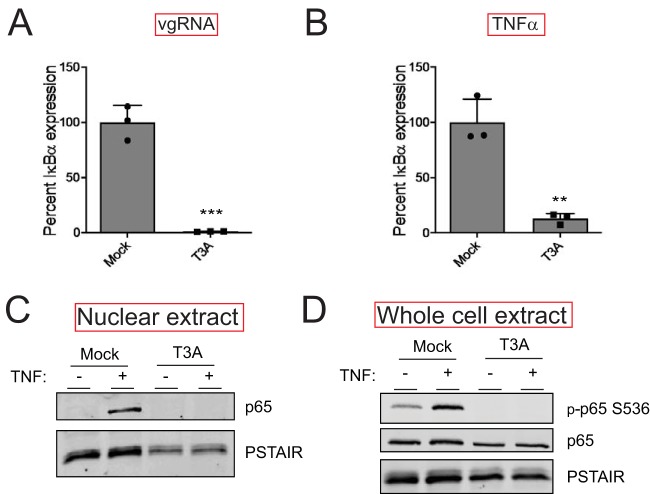
FIG 3.
Reovirus inhibits NF-κB signaling upstream of gene expression. (A) ATCC L929 cells were adsorbed with PBS (mock) or 10 PFU/cell of T3A. Following incubation at 37°C for 20 h, cells were transfected with vgRNA and incubated for 7 h. RNA was extracted from cells, and levels of IκBα mRNA relative to those in a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control were measured using RT-qPCR. IκBα expression in mock-infected cells treated with agonist vgRNA was set to 100%. Gene expression of each replicate, the mean value, and standard deviation (SD) are shown. ***, P < 0.001 by Student’s t test in comparison to mock-infected cells transfected with vgRNA. (B) ATCC L929 cells were adsorbed with PBS (mock) or 10 PFU/cell of T3A. Following incubation at 37°C for 20 h, cells were treated with 10 ng/ml TNF-α and incubated for 1 h. RNA was extracted from cells and levels of IκBα mRNA relative to those in a GAPDH control were measured using RT-qPCR. IκBα expression in mock-infected cells treated with the agonist TNF-α was set to 100%. Gene expression of each replicate, the mean value, and SD are shown. **, P < 0.01 by Student’s t test in comparison to mock-infected cells transfected with vgRNA. (C) ATCC L929 cells were adsorbed with PBS (mock) or 10 PFU/cell of T3A. Following incubation at 37°C for 24 h, cells were treated with 10 ng/ml TNF-α and incubated for 1 h. Nuclear extracts were immunoblotted using antiserum specific for p65 or for PSTAIR loading control. (D) ATCC L929 cells were adsorbed with PBS (mock) or 10 PFU/cell of T3A. Following incubation at 37°C for 24 h, cells were treated with 20 μM proteasome inhibitor PSI for 1 h (to prevent turnover of proteins regulated by TNF-α signaling), then with 10 ng/ml TNF-α for 30 min. Whole-cell extracts were immunoblotted with antisera specific for p65, p65 Ser536 phosphorylation, and PSTAIR.