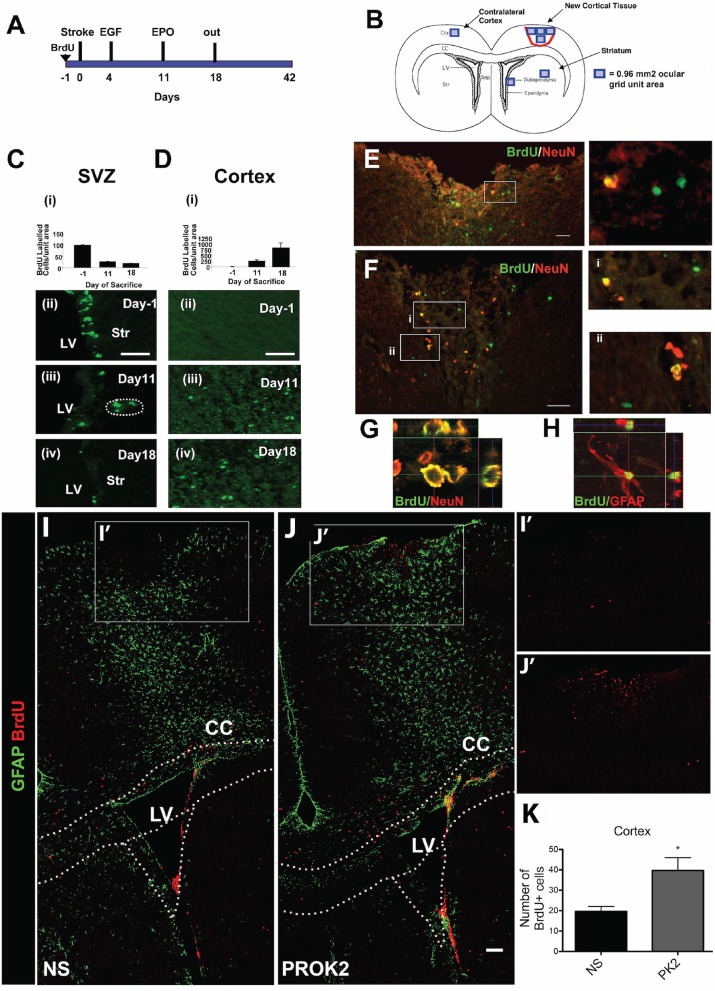
Figure 2.
Pharmacological and signaling peptide approaches to augment endogenous NPC proliferation and migration. (A–H) Intraventricular infusion of EGF followed by EPO and (I–K) injection of recombinant Prokinectin 2 (PROK2) into the injured cortex. (A) Experimental design utilized by Kolb et al. (137). Rats received BrdU injections 1 day prior to stroke, followed by EGF on days 3 and 4 following stroke and EPO on day 11 following stroke. (B) Schematic illustration depicting the four regions (indicated by squares) in which BrdU-positive cells were counted. (C,D) Quantification and corresponding fluorescent images depicting the number of BrdU-positive cells in the SVZ (over the 0.96 mm2 area indicated in B) and cortex (over the 3.84 mm2 area indicated in B) 1 day before stroke (−1) and 11 and 18 days post-stroke following growth factor administration. BrdU-positive cells decreased in the SVZ and increased in the cortex over time. (E,F) Fluorescence images depicting BrdU and NeuN double-positive cells in the injured cortex. Insets depict higher magnification images of NeuN/BrdU cells. Confocal images of (G) BrdU/NeuN double-positive neurons and (H) BrdU/GFAP double-positive astrocytes in the injured cortical tissue. In the experimental design utilized by Mundim et al. (145), mice were administered BrdU for 2 days prior to the injection of either recombinant PROK2 or saline into the cortices of uninjured mice. (I,J) Confocal microscopy images and (K) quantification reveal a greater quantity of BrdU-positive cells (red) in the cortex following PROK2 administration compared to saline (NS). Scale bars (C,D) 50 microns; (E–G) 30 microns; (K) 100 microns. Ctx, cortex; cc, corpus callosum; LV, lateral ventricle; Str, striatum; NS, normal saline. Reprinted with permission from Kolb et al. (137) for (A–H) and Mundim et al. (145) for (I–K). *p < 0.05.