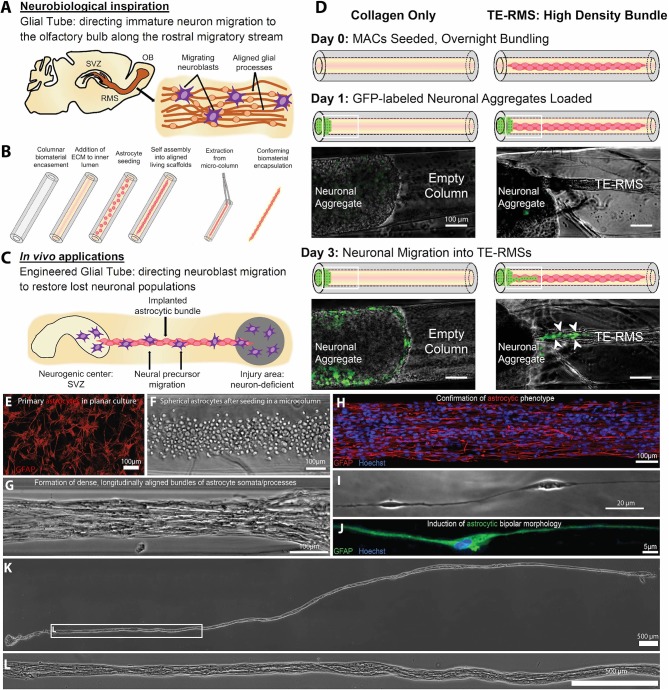
Figure 4.
A tissue-engineered rostral migratory stream (TE-RMS) for directed neuronal replacement following brain injury. Schematic illustration of (A) the rodent rostral migratory stream comprised of aligned astrocytes, (B) the TE-RMS fabrication process, and (C) predicted in vivo applications of this technology. (D) GFP-transduced cortical neuronal aggregates were seeded at one end of hydrogel columns containing either fully formed TE-RMSs or collagen only. Immature neurons migrated out of cortical aggregates and along TE-RMSs, but not along acellular collagen-only columns. White arrows indicate migrating immature neurons. Fluorescence microscopy and phase contrast images showing (E) the GFAP-positive astrocytes with stellate morphology used to fabricate TE-RMSs, (F) the high density of astrocytes shortly after seeding into a collagen-containing microcolumn during TE-RMS fabrication, (G) self-assembly of astrocytes into dense longitudinal bundles during TE-RMS formation, (H) maintenance of astrocyte alignment and bipolar morphology post-extraction from the microcolumn, and (I,J) individual aligned astrocyte processes within microcolumns. (K,L) Phase contrast images demonstrating that centimeter-scale TE-RMSs (>1.5 cm) maintain integrity when extracted from the hydrogel microcolumn. Scale bars (D–H) 100 microns; I: 20 microns; (K,L): 500 microns. Adapted with permission from O'Donnell et al. (40) for (A–D) and Winter et al. (39) for (E–L).