Figure 1.
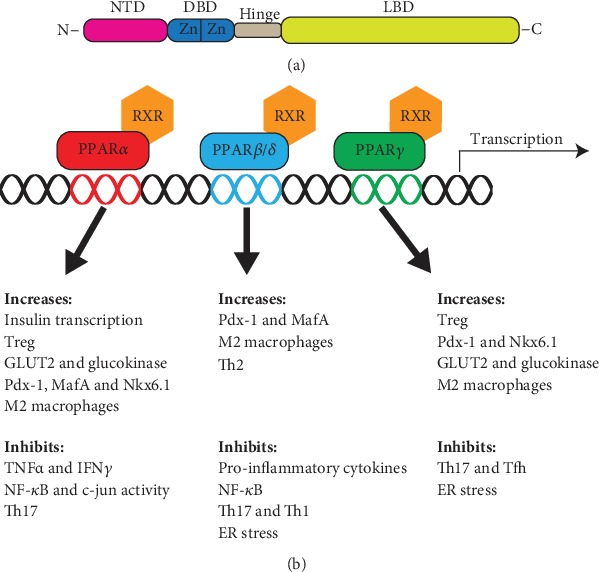
Structure and function of PPARs. (a) The peroxisome proliferator-activated receptor (PPAR) isoforms have a large degree of structural overlap, consisting of an N-terminal ligand-independent transactivation domain (NTD). The DNA-binding domain (DBD) contains two zinc finger (Zn) domains, which bind to peroxisome proliferator response element (PPRE) sequences. The DBD is connected through a hinge domain to the C terminal ligand-binding domain (LBD). (b) Illustration of the biological role of PPARs. PPARs heterodimerize with members of the retinoid X receptor (RXR) family. The isoforms are involved in a variety of pathways; shown are pathways with relation to type 1 diabetes. c-jun: transcription factor c-Jun; GLUT2: glucose transporter 2; MafA: MAF bZIP transcription factor A; NFκB: nuclear Factor-kB; Nkx6.1: NK6 homeobox 1; Pdx-1: pancreatic and duodenal homeobox 1; Tfh: follicular helper T cells; Th1: T helper 1 cells; Th17: T helper 17 cells; Th2: T helper 2 cells; TNFα: tumor necrosis factor alpha; Treg: regulatory T cells.
