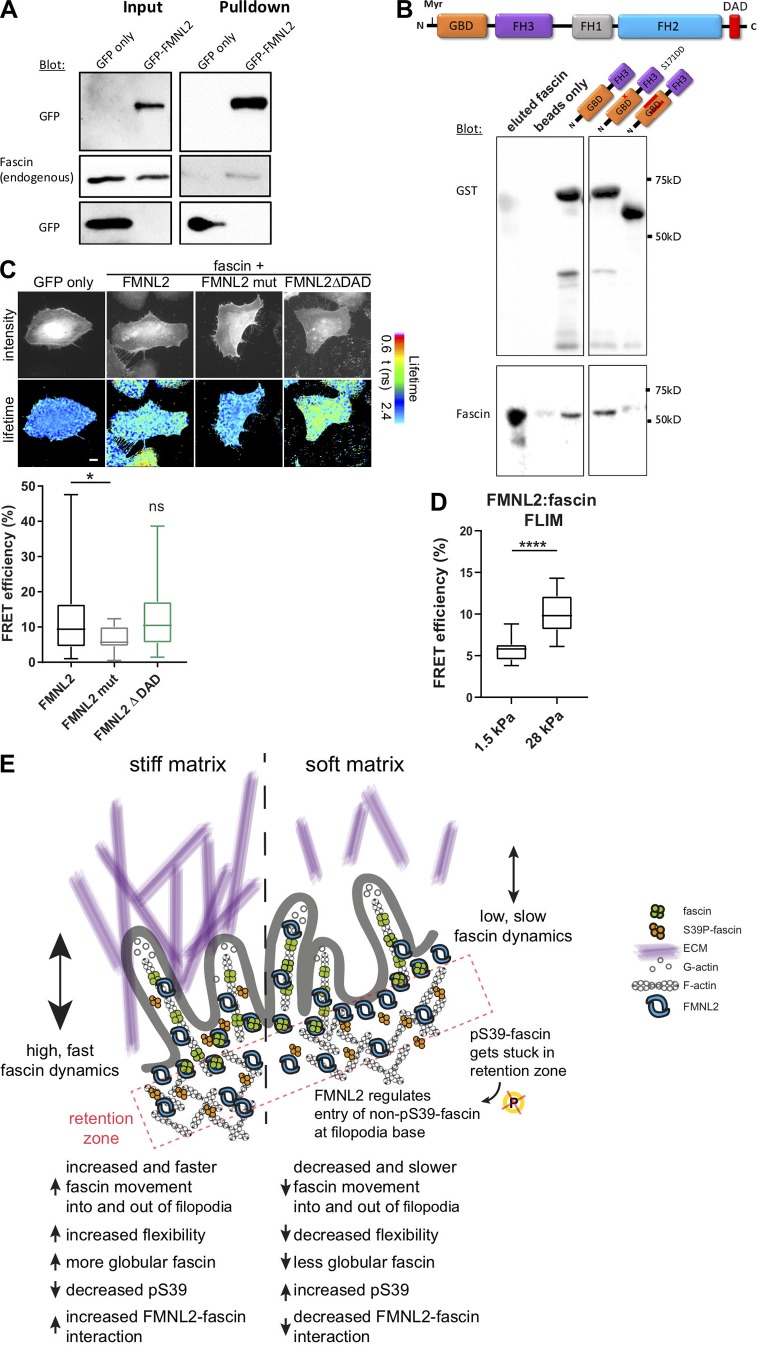
Figure 7.
FMNL2 directly interacts with fascin. (A) GFP trap analysis to evaluate complex formation between FMNL2-GFP and endogenous fascin. FMNL2-GFP was overexpressed in HeLa cells, and GFP trap was performed from cell lysates. Levels of GFP and fascin were evaluated in lysates (input) and pulldowns via Western blot. Representative Western blot of three independent experiments is shown. (B) Direct protein–protein interaction between full-length fascin and FMNL2. Top: Schematic view of full-length FMNL2 with GBD, FH3, FH1, FH2, and DAD domains. Bottom: GST-pulldown experiments of fascin and N-terminal FMNL2 truncations (GBD-FH3, GBD-FH3 with point mutation S171DD) and GBD-FH3 with deletion in GBD domain). GST-tagged FMNL2 constructs and fascin levels were evaluated using Western blot. Representative experiment of three independent experiments is shown. (C) FLIM-FRET lifetime measurement of fixed HeLa cells expressing RFP-fascin and GFP-tagged WT FMNL2, mutant FMNL2 (A272E), and active FMNL2ΔDAD. Intensity images of GFP fluorescence (top panel) and FLIM data in pseudocolor (bottom panel) are shown. Warm colors show high FRET (low GFP lifetime), whereas cool colors indicate low FRET (high lifetime). GFP-fascin alone served as donor-only control and for calculations of FRET efficiency (%). Scale = 5 µm. Calculated FRET efficiency (relative to GFP-fascin alone) for fascin:formin interaction (n = 26, 18, or 21 cells respectively). One-way ANOVA; *, P ≤ 0.05; ns, not significant. (D) FMNL2–fascin interaction on soft (1.5-kPa) and stiff (28-kPa) 2D surfaces were measured with FLIM-FRET. Student t test; ****, P ≤ 0.0001, n = 18 and 17 cells for 1.5 and 28 kPa, respectively. (E) Proposed model. pS39-fascin accumulates at the cell periphery and is subsequently dephosphorylated before it interacts with FMNL2. FMNL2 acts as gatekeeper to control fascin movement into and out of filopodia to allow flexibility and adaptation to the stiffness of the ECM.