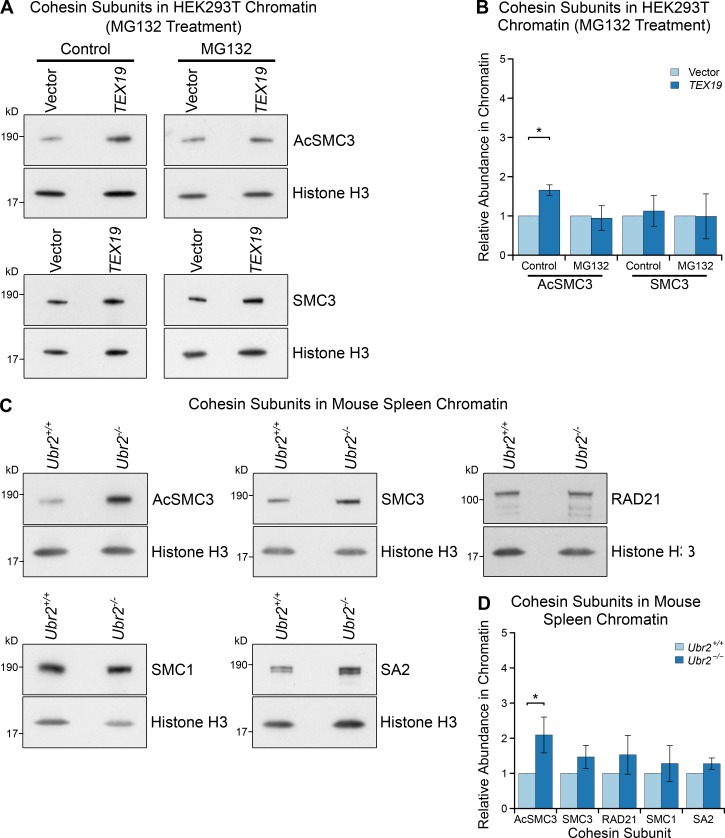
Figure 6.
Proteasome-dependent and Ubr2-dependent pathways regulate AcSMC3-containing cohesin in mammalian somatic cells. (A and B) Representative Western blots (A) and quantification (B) determining the abundance of AcSMC3 and SMC3 cohesin subunits in chromatin from HEK293T cells treated with the proteasome inhibitor MG132. Cells were transfected with either TEX19 or empty vector before treatment with either MG132 or DMSO as a vehicle control. Cohesin abundance was normalized to histone H3 and quantified relative to empty vector transfections. Means ± SD are indicated. Expression of TEX19 induces a 1.6-fold increase in chromatin-associated AcSMC3 (*, t test, P < 0.05; n = 3), but this effect is abolished in the presence of MG132. (C and D) Representative Western blots (C) and quantification (D) from Ubr2+/+ and Ubr2−/− mice determining the abundance of cohesin subunits in spleen chromatin. Cohesin abundance was normalized to histone H3 and quantified relative to Ubr2+/+ mice. Means ± SD are indicated. Ubr2−/− spleens have a 2.1-fold increase in the amount of chromatin-associated AcSMC3 (*, t test, P < 0.05; n = 4).