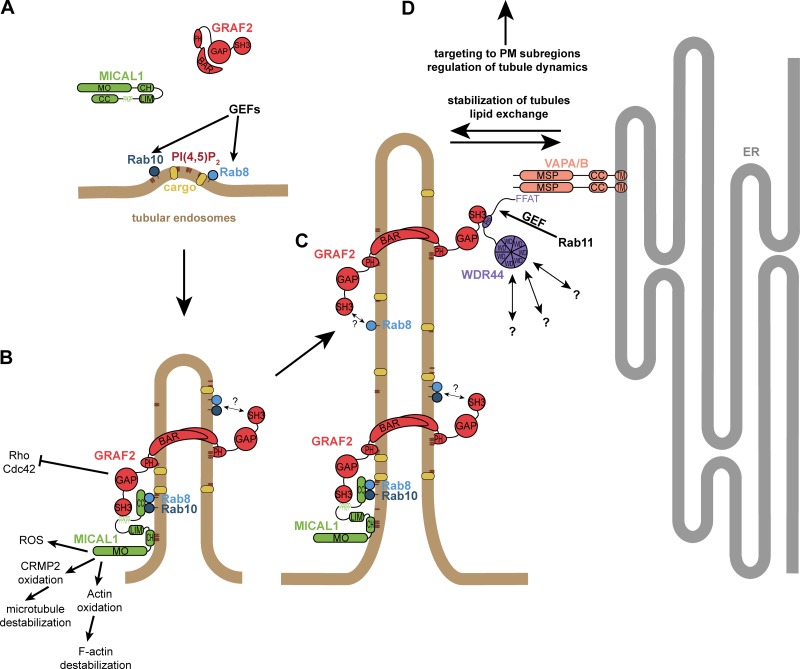
Figure 10.
Working model for the formation of MICAL1/GRAF2/WDR44 tubules. (A) Activation of Rab10 and then Rab8a/b by unknown guanine nucleotide exchange factors (GEFs) promotes their association with budding tubules, which originate in the perinuclear region of cells and could be described as a subset of tubular endosomes. Cargo selection proceeds by an unknown mechanism, which may be related to a specific lipid environment such as an enrichment in PI(4,5)P2 or cholesterol (Ling et al., 2007; Tan et al., 2015; Annabi et al., 2001; Seveau et al., 2004), partitioning in highly curved membranes, direct binding of Rabs or adapters (Ling et al., 2007; Lock and Stow, 2005; Lau and Mruk, 2003), and/or cytoskeletal retention (MacDonald et al., 2018). (B) GTP-bound Rab10 and Rab8a/b recruit and activate MICAL1, which may bind two Rabs simultaneously (Rai et al., 2016; Esposito et al., 2018). MICAL1 may also bind directly to the membrane via its PI(4,5)P2-binding CH domain (Alqassim et al., 2016). MICAL1 can now recruit and activate GRAF2, which by similarity to GRAF1b is expected to directly bind to PI(4,5)P2–containing membranes via its PH domain (Lundmark et al., 2008). The BAR domain of GRAF2 increases its affinity for Rab8/10 membranes and promotes tubule extension. Activated GRAF1b/2 and MICAL1 trigger rearrangements of the cytoskeleton, which may contribute to regulating the stability and dynamics of the tubules. Other unknown proteins (?) may also bridge GRAF2 and Rab8/10. (C) The MO activity of MICAL1 leads to its dissociation from intracellular membranes and allows GRAF2 to recruit WDR44. The association of Rab8 with WDR44-positive tubules is more stable than that of Rab10. (D) WDR44 bridges GRAF2-positive tubules to the ER proteins VAPA/B and, via its WD repeats that fold in a β-propeller, may recruit additional unknown proteins (?) involved in trafficking. See text for further details. GAP, GTPase Activating Protein; ROS, reactive oxygen species; CC, coiled coil; MSP, Major Sperm Protein; TM, transmembrane; PM, plasma membrane.