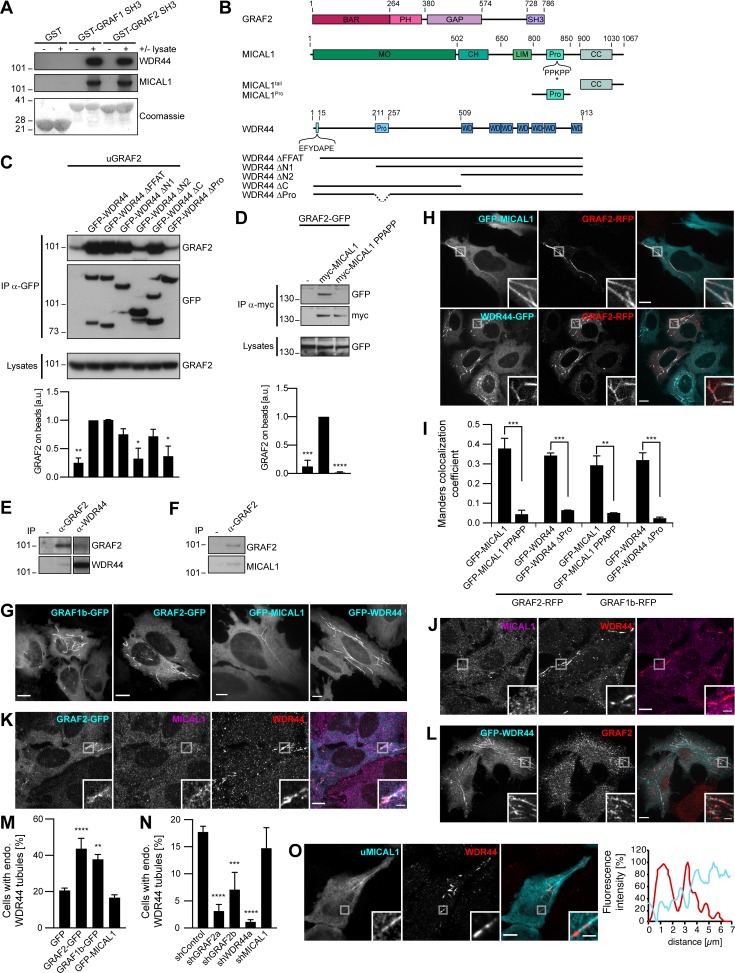
Figure 2.
GRAF1b and GRAF2 bind to MICAL1 and WDR44. (A) Pull-down of HeLa cell proteins by GST, GST–GRAF1 SH3, or GST–GRAF2 SH3, analyzed on two replicate membranes. One was probed with α-WDR44, the other with α-MICAL1. They were then stained with Coomassie, revealing GST-tagged proteins. (B) Schematic representation of human GRAF2, MICAL1, and WDR44 and of mutants used. GRAF2 has two membrane-binding domains, BAR and PH, a GTPase Activating Protein (GAP) domain, and an SH3 domain. MICAL1 has an NADPH-binding MO domain, CH and LIM domains, a proline-rich region with a PPKPP motif, and a C-terminal coiled coil (CC). * indicates K832 mutated in MICAL1 PPAPP. WDR44 has an N-terminal FFAT motif, a proline-rich region, and seven WD repeats. (C and D) Immunoprecipitation (IP) of transfected 293T cells with α-GFP (C) or using α-myc–coated beads (D). The efficiency of GRAF2 binding (untagged GRAF2 [uGRAF2]; C) or GRAF2-GFP (D) was quantified, using coimmunoprecipitation with GFP-WDR44 (C) or myc-MICAL1 (D) as reference. n = 3 or 4. Binding was decreased by removal (C) or mutation (D) of proline-rich regions. (C) Note that GFP-WDR44 underwent proteolysis. (E) Endogenous WDR44 and GRAF2 were coimmunoprecipitated from a HeLa cell lysate using α-GRAF2 and α-WDR44, but not without antibody (−). The membrane was probed with α-GRAF2, followed by α-WDR44. The left and right parts of the membrane were treated identically. (F) Endogenous MICAL1 was coimmunoprecipitated from an SH-SY5Y cell lysate using α-GRAF2, but not without antibody (−). The membrane was probed with α-GRAF2, followed by α-MICAL1. (G) Confocal stacks of transfected HeLa cells. GFP-tagged GRAF1b, GRAF2, MICAL1, and WDR44 were cytosolic and associated with intracellular tubules. (H) Confocal images of transfected HeLa cells. GRAF2-RFP colocalized with GFP-tagged MICAL1 and WDR44. (I) Manders colocalization coefficients for GFP-tagged MICAL1 and WDR44 proteins on GRAF1b/2-RFP–associated structures. n = 15–30 cells. (J–L) Confocal images of HeLa cells stained with α-MICAL1 and α-WDR44 (J and K) or α-GRAF2 (L). (J) Untransfected cells had distinct WDR44 tubules but showed a diffuse distribution of MICAL1. (K) Endogenous WDR44 and to a lesser extent endogenous MICAL1 colocalized with GRAF2-GFP. (L) Endogenous GRAF2 was found on GFP-WDR44 tubules. (M and N) Percentage of transfected HeLa cells with endogenous (endo.) WDR44 tubules. (M) n = 4–8. (N) n = 3–8. (O) Confocal images of transfected HeLa cells stained with α-WDR44 and fluorescence intensity profiles along the tubule enlarged in the boxed area showing complementary distribution of untagged MICAL1 (uMICAL1, cyan line) and endogenous WDR44 (red line) on the same tubule. (C, D, I, M, and N) Data are means ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001. (G, H, J–L, and O) Insets show magnifications of the boxed areas. Scale bars: 10 µm; scale bars of insets: 2 µm.