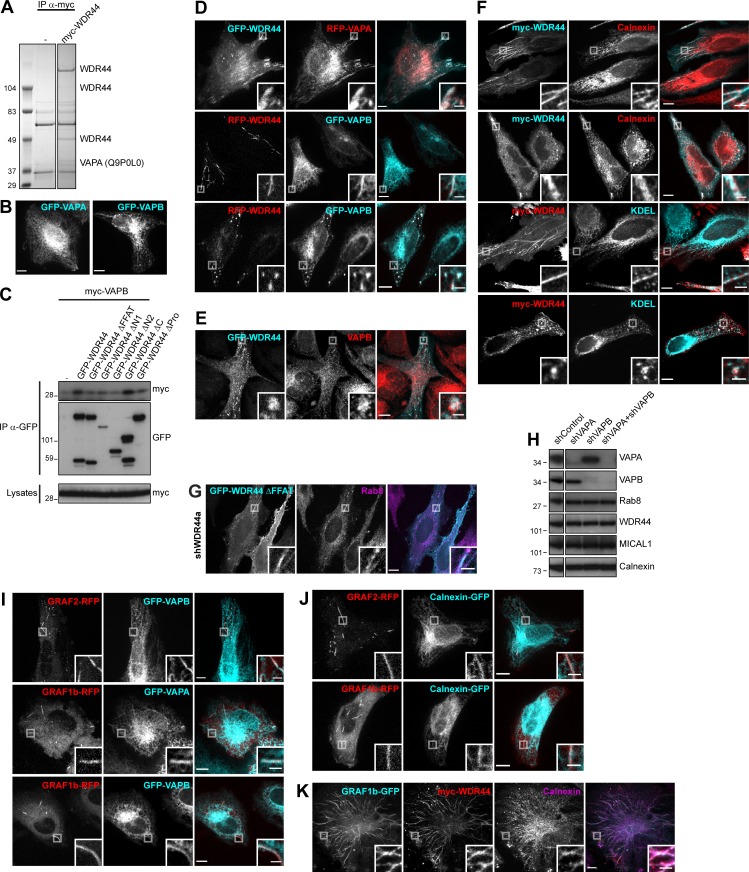
Figure S6.
WDR44 tubules are in close contact with the ER via binding to VAPA/B. (A) Immunoprecipitation (IP) of endogenous proteins from 293T cells, untransfected or expressing myc-WDR44, with α-myc. Bound proteins were analyzed by electrophoresis and Coomassie staining. VAPA (Uniprot accession no. Q9P0L0) was identified by LC-MS/MS. (B) Confocal images of transfected HeLa cells showing GFP-VAPA or GFP-VAPB in reticular ER. (C) Immunoprecipitation (IP) of transfected 293T cells with α-GFP. myc-VAPB was coimmunoprecipitated by GFP-WDR44 but not by mutants lacking the first 14 aa of the protein. (D) Confocal images of transfected HeLa cells showing colocalization of WDR44 and VAPA/B on tubules and on peripheral patches. (E) Confocal images of transfected HeLa cells stained with α-VAPB showing colocalization of GFP-WDR44 patches and endogenous VAPB. (F) Confocal images of transfected HeLa cells stained with α-myc and α-Calnexin or α-KDEL. Myc-WDR44 tubules and patches colocalized with Calnexin, an ER transmembrane protein, and α-KDEL, which recognizes luminal ER proteins containing the ER retention signal KDEL. (G) Confocal images of shWDR44a-expressing HeLa cells transfected with GFP-WDR44 ΔFFAT and stained with α-Rab8. GFP–WDR44 ΔFFAT was associated with Rab8-positive tubules. (H) Western blot analysis of an equal protein amount of shRNA-expressing HeLa cell lysates showing specific knockdown of VAPA in shVAPA- and of VAPB in shVAPB-transfected cells. Calnexin was used as loading control. The two parts of the blots were from the same membrane and correspond to identical exposure times. The membrane was initially probed with α-WDR44 and α-VAPB, followed by α-MICAL1 and α-VAPA, and finally α-Calnexin and α-Rab8. (I and J) Confocal images of transfected HeLa cells. GRAF1b/2–RFP tubules colocalized with GFP-VAPA/B (I) but rarely with Calnexin-GFP (J). (K) Confocal images of transfected HeLa cells stained with α-myc and α-Calnexin. When WDR44 was coexpressed, GRAF1b tubules colocalized with an endogenous ER marker. (B, D–G, and I–K) Insets show magnifications of the boxed areas. Scale bars: 10 µm; scale bars of insets: 2 µm.