Table 2.
Cross reactivity of the obtained mAb with 1-NAP related compounds (n=3)
| Compound | Structure | IC50 (ng/mL) | CR (%)a |
|---|---|---|---|
| 1-naphthol | 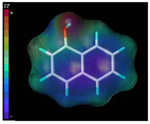 |
12.5 | 100 |
| 2-naphthol | 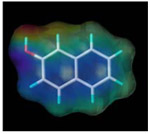 |
1005.0 | 1.2 |
| Carbaryl | 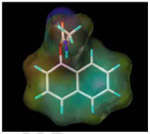 |
300.7 | 4.2 |
| 1, 3-dihydroxy naphthalene | 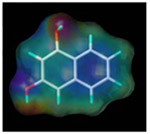 |
178.0 | 6.9 |
| 1, 4-dihydroxy naphthalene | 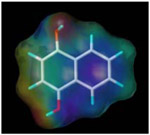 |
200.7 | 6.2 |
| 1, 5-dihydroxy naphthalene | 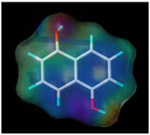 |
87.4 | 14.3 |
| 1, 6-dihydroxy naphthalene | 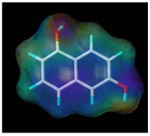 |
502.8 | 2.5 |
| 1, 7-dihydroxy naphthalen | 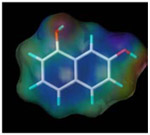 |
881.7 | 1.4 |
| 1, 8-dihydroxy naphthalene |  |
4303.4 | 0.3 |
| 2, 6-dihydroxy naphthalene |  |
2839.7 | 0.4 |
CR, cross-reactivity. The percentage of CR was calculated using the following equation: CR (%) =[IC50 (NAP)/IC50 (cross-reactant)] × 100.
