Table 1.
Reaction conditions for the direct decarboxylative alkylation (DDA) of anilines.[a]
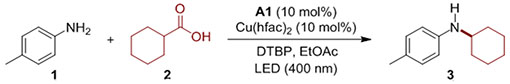 | ||
|---|---|---|
| Entry | Change from optimal conditions | Yield, %[b] |
| 1 | No change | 99 (97[c]) |
| 2 | No light | 0 |
| 3 | No A1 | 0 |
| 4 | No Cu(hfac)2 | 0 |
| 5 | No DTBP | 7 |
| 6 | A2 instead of A1 | 86 |
| 7 | Cu(acac)2 instead of Cu(hfac)2 | 38 |
| 8 | PhCF3 instead of EtOAc | 78 |
| 9 | PhCH3 instead of EtOAc | 76 |
| 10 | 420 nm instead of 400 nm LED | 86 |
| 11 | Under air | 94 |
Reaction conditions: aniline (0.3 mmol), carboxylic acid (0.75 mmol), A1 (10 mol%), Cu(hfac)2 (10 mol%), DTBP (0.6 mmol), EtOAc (4.5 mL), LED (400 nm), 12 h.
Determined by 1H NMR with 1,4-dimethoxybenzene as an internal standard.
Isolated yield.
