Figure 7. D1R mutants cause oxidative stress.
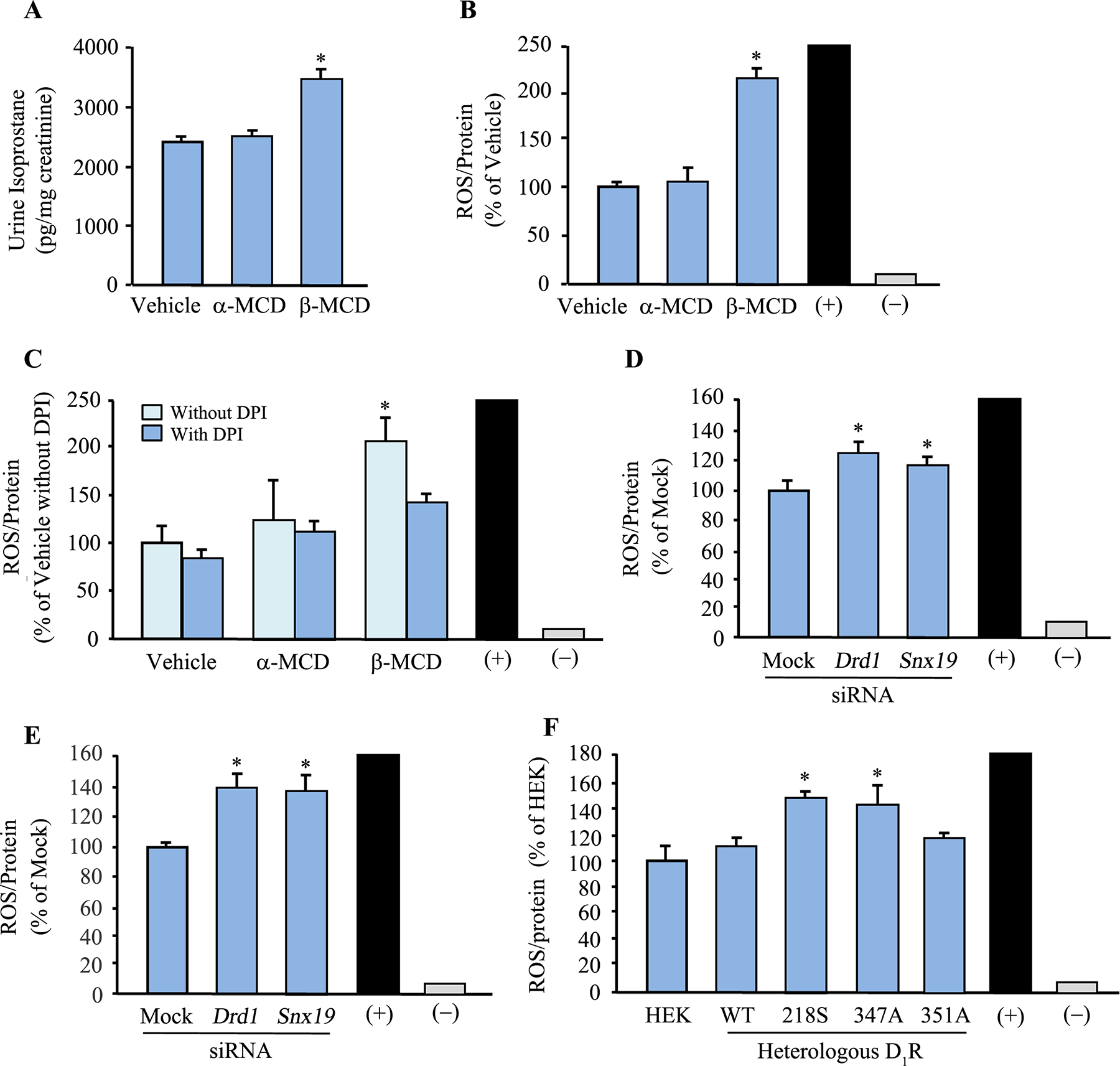
A: Urine isoprostanes were measured and normalized for urine creatinine to determine the presence of oxidative stress in uninephrectomized adult male C57BL/6J mice on normal salt (0.8% NaCl) diet that received a 7-day renal-restricted lipid raft disruption via a renal subcapsular minipump infusion of the lipid raft disruptor β-MCD. α-MCD and vehicle were used as controls. (+) = 1% H2O2, (–) = without ROSstart. B and C: ROS production in mouse RPTCs treated with β-MCD, or α-MCD and vehicle as controls, for 2 hr. ROS was measured using ROSstart™ 650. In another set of experiments, diphenyleneiodonium (DPI; a NOX inhibitor) was used to determine if the increased ROS production was due to enhanced NOX activity. *P<0.05, vs. vehicle, α-MCD, or with DPI, one-way ANOVA and Holm-Sidak post-hoc test, n=3/group. (+, positive control, 200–250 ± 10% vs. vehicle) = 1% H2O2, (-, negative control) = without ROSstart. D and E: ROS production in mouse (D) and human (E) RPTCs in which DRD1 or SNX19 was silenced using siRNA. Non-silencing “Mock” siRNA was used as control. ROS was measured via ROSstart™ 650. *P<0.05, vs. Mock, one-way ANOVA and Holm-Sidak post-hoc test, n=3/group. (+, positive control, 200–250 ± 10% vs. vehicle) = 1% H2O2, (-, negative control) = without ROSstart. F: ROS production in HEK-293 cells transfected with human D1R-WT or mutants for 72 hrs. ROS was measured with ROSstart™ 650. *P<0.05, vs. untransfected HEK293 (HEK), human D1R-WT (WT), or ΔD1R-351A (351A), one-way ANOVA and Holm-Sidak post-hoc test, n=3/group. (+, positive control, 200–250 ± 10% vs. vehicle) = 1% H2O2, (-, negative control) = without ROSstart
