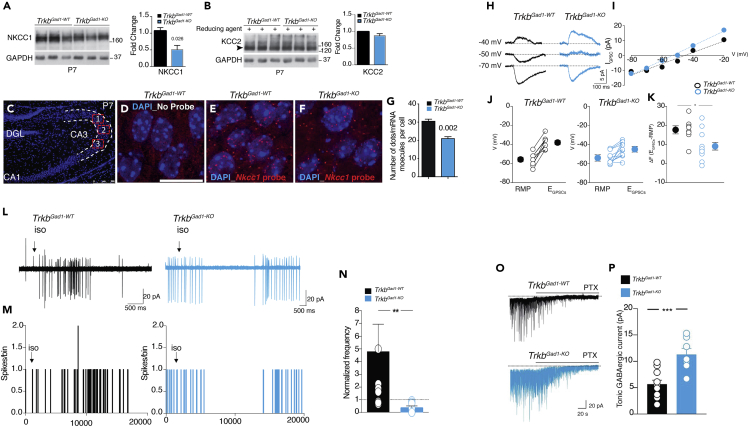
Figure 4.
Reduced Expression of Nkcc1 and Altered Direction of GABA Action at Immature MF-CA3 Synapses in TrkbGad1-KO Mice
(A and B) Representative western blots from P7 hippocampal lysates and relative quantification of NKCC1 protein levels (P7, TrkbGad1-WT [n = 6], 1.065 ± 0.1013; TrkbGad1-KO [n = 6], 0.5086 ± 0.1261, p = 0.026) (A) and KCC2 monomer levels (P7, TrkbGad1-WT [n = 6], 0.9846 ± 0.01889, TrkbGad1-KO [n = 6], 0.8694 ± 0.06086, p = 0.1) (B). GAPDH, loading control.
(C–G) Single-molecule fluorescence in situ hybridization (smFISH) was used to detect and count individual Nkcc1 RNA molecules in single cells of the CA3 region at P7. (C) Representative image of a P7 hippocampal section stained with DAPI. Highlighted are three random fields imaged in the CA3 region for quantification of single-molecule RNA (more details in Transparent Methods). (D–F) Representative images from the CA3 regions of control and mutant mice highlighting the single cells by DAPI nuclear staining. The red spots corresponding to single mRNA molecules derived from the transcription of Nkcc1 are detected with the Quasar570 fluorophore-labeled oligonucleotide probe library in single cells of the CA3 hippocampal region (probe details in Table S4) (E and F); no probe control (D). (G) Quantification of single mRNA molecules per cell (TrkbGad1-WT, 30.67 ± 0.9943 from n = 376 cells; TrkbGad1-KO, 21.16 ± 0.8630 from n = 396 cells, p = 0.002; n = 3 P7 pups each genotype). DGL, dentate granule layer. Scale bars: 250 μm in (C) and 50 μm in (D–F).
(H–K) Reduced driving force for GABA-mediated postsynaptic currents (GPSCs) at MF-CA3 synapses in TrkbGad1-KO mice. (H) Representative traces of GPSCs evoked at three different holding potentials in CA3 principal cells by MF stimulation (gramicidin-perforated patches) in TrkbGad1-WT and TrkbGad1-KO mice. (I) Amplitudes of GPSCs (IGPSC) shown in (H) are plotted against holding potentials (V). (J) Individual RMPs and EGPSCs values in CA3 principal cells from control (n = 8 from 5 pups) and TrkbGad1-KO (n = 11 from 7 pups). Larger symbols on the left and right refer to mean ± SEM values (RMPs, TrkbGad1-WT, −55.6 ± 2.4 mV; TrkbGad1-KO, −54.2 ± 2 mV; TrkbGad1-WTEGPSCs, −37.9 ± 2.3mV; TrkbGad1-KO, −44.9 ± 2.3mV). (K) Plot of the driving force (ΔF) for GABA (ΔF=EGPSCs–RMP) in individual experiments from TrkbGad1-WT and TrkbGad1-KO mice. Larger symbols are mean ± SEM values. ΔF= 17.7 ± 2.1mV in TrkbGad1-WT and 9.1 ± 2.2mV in TrkbGad1-KO mice; ∗p = 0.03, Wilcoxon test.
(L–N) Altered GABAergic signaling accounts for network dysfunction in TrkbGad1-KO immature hippocampus. Effects of isoguvacine on spontaneous firing of CA3 principal cells in TrkbGad1-WT and TrkbGad1-KO mice. (L) Two representative examples of changes in spontaneous firing induced by pressure application of isoguvacine (100 μM for 1 s; arrows) to CA3 principal cells (recorded in cell-attached) in TrkbGad1-WT (black) and TrkbGad1-KO mice (cyan). (M) Interspike interval histograms (bin: 10 ms) for cells shown in (L). (N) Summary plot showing isoguvacine-induced changes in spike frequency (30 s after drug application) normalized to baseline values (30 s before drug application); TrkbGad1-WT, 4.78 ± 2.1 (n = 9 from 4 pups), TrkbGad1-KO, 0.39 ± 0.1 (n = 8 from 5 pups); ∗∗p = 0.025. Values are mean ± SEM, p statistic from unpaired Student's t test.
(O and P) Increased GABAA-mediated tonic conductance in TrkbGad1-KO mice. (O) Representative traces of spontaneous GABAA-mediated synaptic currents (sGPSCs) recorded from CA3 principal cells before and during application of picrotoxin (PTX, 100 μM bars above the traces) in hippocampal slices obtained from control (n = 11) and TrkbGad1-KO mice (n = 7). Note the upward shift of the baseline current and disappearance of sGPSCs after application of PTX (100 μM) in the presence of DNQX (20 μM) and DL-APV (100 μM). (P) Each column represents the mean tonic GABAA-mediated conductance measured in controls, 5.7 ± 0.75 pA (n = 11) and TrkbGad1-KO mice, 11.3 ± 1.2 pA (n = 7). ∗∗∗p = 0.0008, Mann-Whitney test.
See also Figures S5 and Table S4.