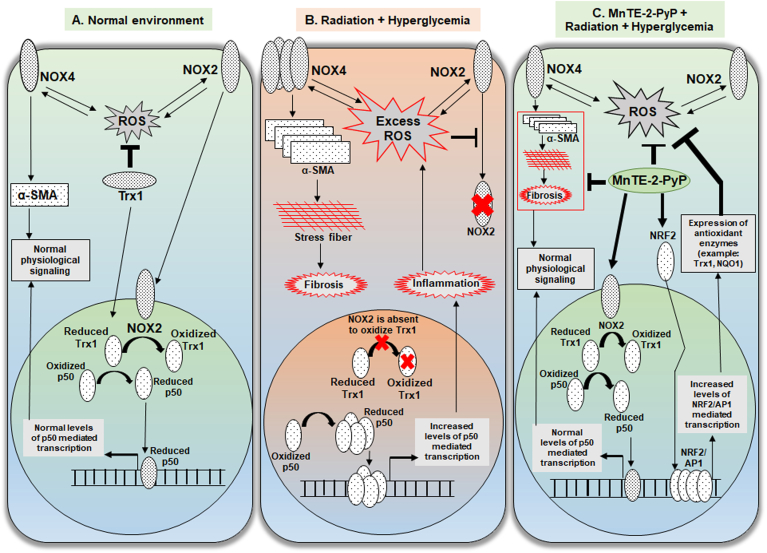
Fig. 8.
Graphical summary of MnTE-2-PyP mediated normal prostate fibroblast protection mechanism. A. In the normal cellular environment, a basal physiological level of ROS is produced by NOX4 and NOX2 by a positive feedback loop. NOX4 expression positively regulates the expression α-SMA, which maintains the normal levels of cellular elasticity. Two redox modulators, NOX2 and Trx1 translocate to nucleus. NOX2 oxidizes Trx1 that limits the availability of reduced Trx1 in the nucleus to reduce p50 subunit of NFκB. Reduced p50 binds to DNA to maintain NFκB-p50 mediated normal survival response. B. In an irradiated and hyperglycemic cellular environment, excess levels of ROS produces increased levels of NOX4 followed by increased levels of α-SMA, which induces pro-fibrotic signaling and accumulates stress fibers in the cell that results in fibrosis. Excess levels of ROS inhibits nuclear localization of NOX2. Absence of NOX2 in the nucleus allows Trx1 mediated increased reduction of p50 and increased DNA binding. This results in an increased inflammatory response in the cell. C. In the irradiated and hyperglycemic cellular environment, MnTE-2-PyP directly reduces ROS and also promotes nuclear translocation of NOX2. Nuclear NOX2 inhibits p50 DNA binding by limiting its reduction by Trx1. MnTE-2-PyP increases expression of NRF2 and transcriptional activity of NRF2/AP1. NRF2/AP1 mediated increased expression of antioxidant enzymes inhibit the excess ROS load in the cell.