Figure 1. Protein associations of FKBP51 impact PTMs.
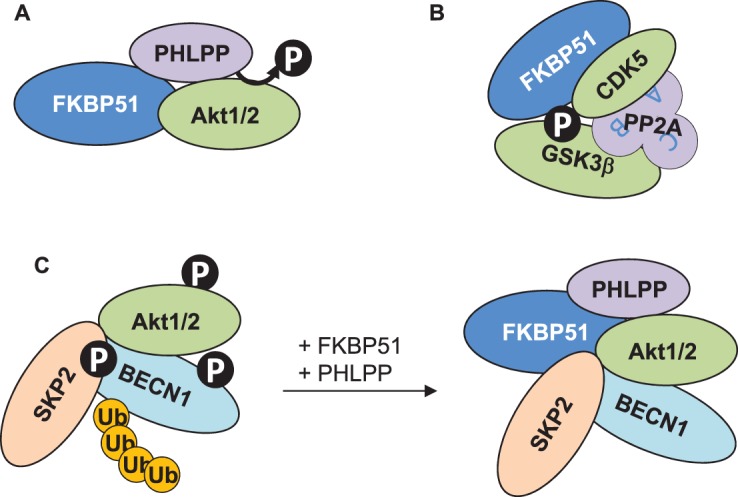
(A) FKBP51 associates with the phosphatase PHLPP and the kinase Akt, which leads to de-phopshporylation and thus inactivation of Akt [57,58]. (B) The association of FKBP51 with CDK5, GSK3β and PP2A (with the subunits A–C) presumably results in increased phosphorylation of GSK3β [67]. (C) Through association with PHLPP, Akt, SKP2 and BECN1, FKBP51 changes two types of PTMs of BECN1, phosphorylation and ubiquitination [58,64,65]. The recruitment of PHLPP leads to lower Akt phosphorylation and activity, entailing less phosphorylation of BECN1 and of the E3-ligase SKP2. Thereby, SKP2 is less active resulting in lower ubiquitination of BECN1. Whether or not FKBP51 associates with all these proteins in one complex remains to be elucidated.
