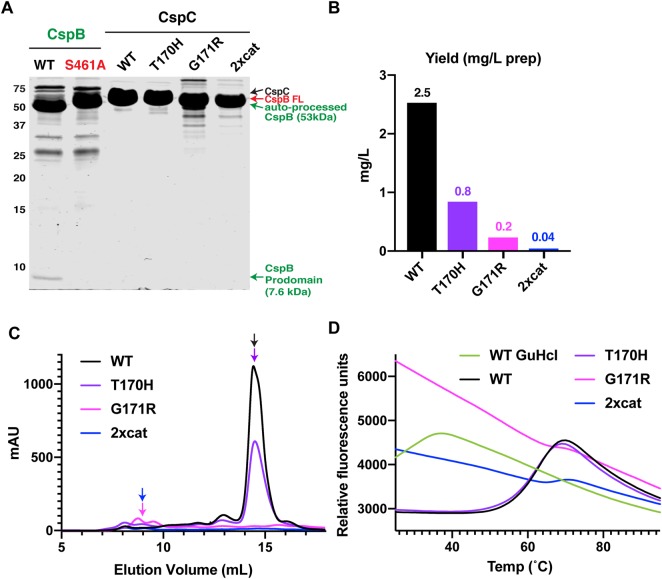
Figure 3. Changes to CspC's pseudoactive site region decrease its stability.
(A) Coomassie stain of purified C. difficile CspB and CspC. WT CspB and its catalytic site mutant, S461A, were affinity purified, while WT CspC and the CspC variants, T170H, G171R, and T170H-G485S (2xcat), were affinity purified using the self-cleaving CPD tag followed by gel filtration. WT CspB autoprocesses to release its 7.6 kDa N-terminal prodomain [27]; the catalytic mutant, CspBS461A, does not undergo autoprocessing (CspB-FL indicates full-length CspB). An active CspC2xcat would be expected to release a 7.5 kDa prodomain, but this is not observed. CspC2xcat and CspCG171R both run at slightly lower apparent MW than WT CspC, since these variants likely undergo CPD-induced trimming of their C-termini, and the C-terminal His-tagged variants run at the same apparent MW as WT CspC (Supplemental Figure S2). (B) Yields of CspC variants following CPD-mediated affinity purification and size exclusion chromatography per liter of culture. (C) Size exclusion chromatography elution profiles for CspC variants measured by mAU (A280). CspC variants purified using the CPD self-cleaving tag from 1 L of culture were concentrated and loaded onto a Superdex 200 Increase 10/300 GL column. (D) Thermofluor melt curves of the proteins purified in (C). GuHCl (guanidine hydrochloride) was used to denature WT CspC.