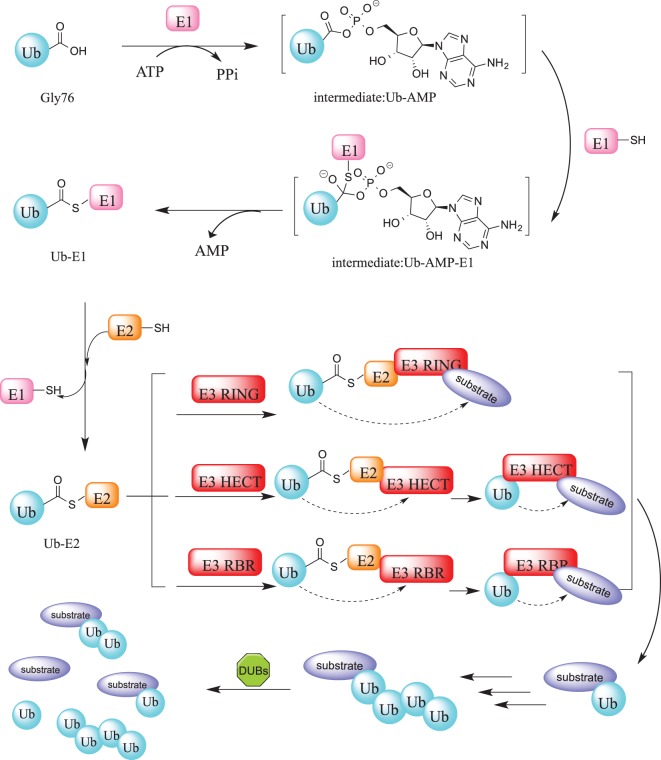
Figure 1. The ubiquitination and deubiquitination cascade.
Protein ubiquitination is initiated by the E1 enzyme that catalyzes the adenylation of the C-terminal glycine residue of Ub, forming Ub-AMP. This phosphoester bond subsequently undergoes a nucleophilic substitution involving the sulfhydryl group of the E1 active site cysteine residue. Following this, Ub is transferred to an E2 enzyme active site cysteine residue through a trans thioesterification reaction that yields an activated thioester-linked E2-Ub complex. Finally, an E3 ligase catalyzes the transfer of Ub from the E2 enzyme to a target substrates creating an isopeptide bond between the C-terminal glycine of ubiquitin and the substrate lysine. Depending on the particular class of E3 ligase, this reaction is stepwise (HECT or RBR E3s) or direct without the formation of intermediates (RING E3s). The ubiquitination process is reversed by DUBs through cleavage of ubiquitin from the substrate.