Table 1.
Condition Optimization
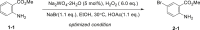 | |||
|---|---|---|---|
| Entry | Vary from Optimized Condition | Yielda | Major By-products |
| 1 | None | 78%–83% | 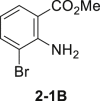 |
| 2 | Without HOAc | <50% conversion for 3 days | |
| 3 | Without Na2WO4-2H2O | Traceb | |
| 4 | H3O40PW12-xH2O instead of Na2WO4-2H2O | 64%c | |
| 5 | (NH4)10(H2W12O42)-xH2O instead of Na2WO4-2H2O | 77%c | 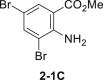 |
| 6 | LiBr instead of NaBr | 66%d | |
| 7 | KBr instead of NaBr | 75%d | |
| 8 | SPB instead of H2O2 | N.R | |
| 9 | MeOH instead of EtOH | 67% | 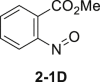 |
| 10 | H2O instead of EtOH | 67% | |
| 11 | Na2WO4-2H2O, 1 mol % instead of 5 mol % | 67% | |
| 12 | 2.0 equiv H2O2 instead of 6.0 equiv | 57% | |
N.R, no reaction
All the reactions were conducted in 1.0-mmol scale (1-1) for 12 h, isolated yield.
2.0 equivalents of HOAc was utilized.
1.5 equivalents NaBr and 2.0 equiv. HOAc were utilized.
1.5 equivalents of NaBr and 2.0 equivalents of HOAc were utilized, and the reactions were conducted at 50°C
