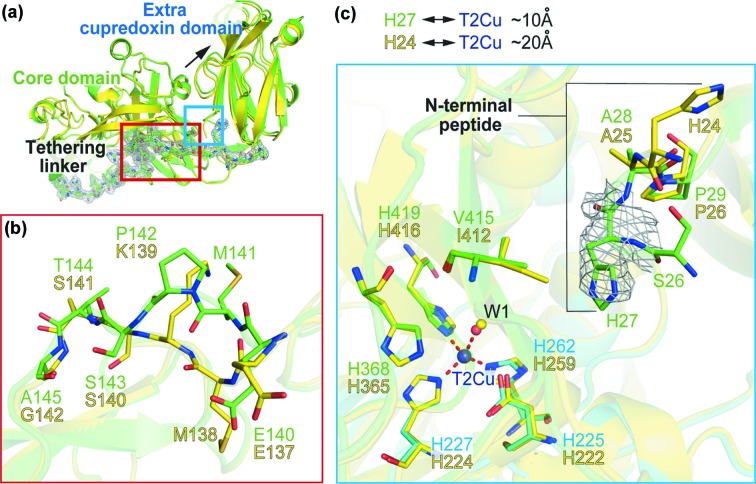
Figure 2.
Structural differences in the tethering linker and N-terminal peptide between Hd 1NES1NiR and Hd A3151NiR. (a) Monomer of Hd 1NES1NiR colored green superimposed on the core domain of Hd A3151NiR colored yellow. The monomer is constructed with the core domain, extra cupredoxin domain and tethering linker between them. The 2F o F c electron-density map at the 1.0σ level is shown for the tethering linker. The main-chain structural difference between the two HdNiRs is indicated by a black arrow. (b) The middle part of the tethering linker and (c) the N-terminal peptide near the T2Cu of Hd 1NES1NiR colored green superimposed on the core domain of Hd A3151NiR coloured yellow. The T2Cu ion in the core domain is represented by a deep-blue sphere. The ligand water (W1) molecules for Hd 1NES1NiR and Hd A3151NiR are represented by red and yellow spheres, respectively. Coordination to the T2Cu ion is shown by a red broken line. The 2F o F c electron density map at the 1.0σ level is shown for the His27 of Hd 1NES1NiR. The distances between His27 and His24 and T2Cu are indicated (∼10 and ∼20 Å for 1NES1 and A3151, respectively).