Table 2.
Alkyne Partner Substrate Scopea
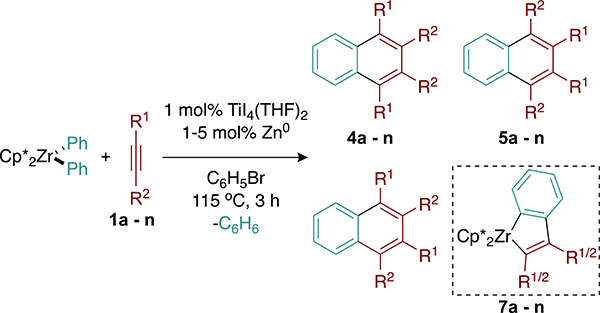 | |||||
|---|---|---|---|---|---|
| R1, R2 | % yield 4:5:6b | % yield 7c | % yield trimerd | A valuee | |
| 1a | Et, Et | 5 | 86 | 8 | 3.5 |
| 1b | nPr, nPr | 4 | 88 | 9 | 3.6 |
| 1c | TMS, H | 0 | >95 | 0 | 2.5 |
| 1d | Ph, H | 0 | >95 | 0 | 3.0 |
| 1e | iPr, Me | 0 | >95 | 0 | 3.9 |
| 1f | Ph, Me | 0 | >95 | 0 | 4.7 |
| 1g | Ph, nBu | 0:4:10 | 19 | 16 | 4.8 |
| 1h | Ph, Ph | 71 | 0 | 24 | 6.0 |
| 1i | p-CF3Ph,p-CF3Ph | 64 | 0 | 19 | 6.0 |
| 1j | p-OMe-Ph,p-OMe-Ph | 78 | 0 | 15 | 6.0 |
| 1k | p-tBuPh,p-tBuPh | 76 | 0 | 22 | 6.0 |
| 1l | Ph, TMS | 8:14:26 | 0 | 0 | 5.5 |
| 1m | nBu, TMS | 0:34:31 | 0 | 0 | 4.3 |
| 1n | Me, tBu | 0 | 0 | 0 | 6.6 |
Reaction conditions: metallocene (0.05 mmol), alkyne (0.05 mmol), catalyst (0.0005 mmol of Ti), Zn0 (0.0005 mmol), 0.5 mL of C6H5Br, 115 °C, 3 h. Cp* = pentamethylcyclopentadiene.
Determined by quantitative GC-FID with respect to an internal standard (1,3,5-trimethoxybenzene).
Determined by 1H NMR with respect to an internal standard (1,3,5-trimethoxybenzene).
% yield of all alkyne cyclotrimer regioisomers; determined by 1H NMR with respect to an internal standard (1,3,5-trimethoxybenzene).
Sum of both alkyne substituent A values.
