Table 2.
Substrate Scope of One-Pot In Situ Pyrazole Synthesis from Alkynes and Nitrilesa
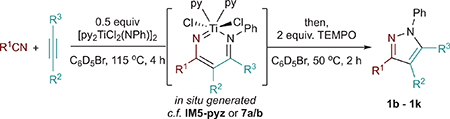 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | product | yield (%) |
| 1b | 4-Me-C6H4 | Et | Et | 1 | 75c |
| 2b | Ph | Et | Et | 1b | 79c |
| 3 | Ph | Et | Et | 1b | 56 |
| 4 | 4-MeO-C6H4 | Et | Et | 1c | 52 |
| 5 | 4-CF3-C6H4 | Et | Et | 1d | 49 |
| 6 | iPr | Et | Et | 1e | 54 |
| 7 | Me | Et | Et | 1f | 43 |
| 8 | Me | Ph | Me | 1g | 28 |
| 9 | Ph | Ph | Me | 1h | 54 |
| 10 | Ph | Me | Me | 1i | 52 |
| 11 | Ph | 4-t-BuC6H4 | 4-t-BuC6H4 | 1j | 37 |
Conditions: 0.025 mmol of [py2TiCl2(NPh)]2, 0.05 mmol (2 equiv) of alkyne, 0.05 mmol (2 equiv) of nitrile, 0.5 mL of PhBr, 115 °C, 4h; yields with respect to alkyne are determined in situ via 1H NMR against internal 1,3,5-trimethoxybenzene standard.
Conditions: 0.025 mmol of [py2TiCl2(NPh)]2, 0.15 mmol (6 equiv) of alkyne, 0.15 mmol (6 equiv) of nitrile, 0.5 mL of PhBr, 115 °C, 1 h.
Yields reported with respect to [py2TiCl2(NPh)]2, which is the limiting reagent under condition B.
