Abstract
SCN2A mutations can cause early-onset epilepsy. Thompson et al. examined these human mutations in neonatal versus adult channel isoforms.
The advancement of whole-genome and -exome sequencing has helped to identify de novo genetic alterations in patients with early-onset epilepsy encephalopathy (EOEE; Sugawara et al., 2001; Heron et al., 2002). However, the evidence that the mutations are indeed pathogenic or how they affect the protein biology and/or neuronal network has not always come hand-in-hand with the genetic discoveries. In a recent issue of JGP, Thompson et al. addressed this issue by determining the electrophysiological and neuronal effects of human mutations occurring in the voltage-activated NaV1.2 (SCN2A) sodium channel (Thompson et al., 2020). They examined the effects of the mutations on the function of the channel itself and integrated these results into the context of neuronal firing or activity. They combined an interdisciplinary—empirical, and computational modeling—approach to conclude that these mutations result in a gain-of-function change, and that their effects are more pronounced when occurring in the neonatal channel isoform compared with the adult isoform of the channel. This work is of interest to physicians and scientists working in the epilepsy field as well as to those interested in NaV1.2 channelopathies and to patients and family of those affected by the disease.
EOEEs affect one to two newborns out of every 1,000 births and are some of the most debilitating and consequential seizures to recur in human infants (Camfield et al., 1996). These patients have a grave prognosis with debilitating outcomes, particularly for intellectual and motor development (Engel, 2001). Therapeutics are available but vary in efficacy, in part because of the diverse etiology and complex physiology underlying different seizures. Clinicians have employed whole-genome and -exome sequencing to identify putatively pathogenic mutations occurring in those affected. SCN2A mutations have been identified in patients diagnosed with Ohtahara syndrome (OS; Nakamura et al., 2013; Wolff et al., 2017; Turkdogan et al., 2019), epilepsy of infancy with migrating focal seizures (EIMFS; Howell et al., 2015; Wolff et al., 2017; Su et al., 2018), and benign familial neonatal infantile syndrome (BFNIS; Misra et al., 2008; Scalmani et al., 2006; Liao et al., 2010b; Xu et al., 2007; Lauxmann et al., 2013), among other early-onset or childhood epilepsies (Liao et al., 2010a; Schwarz et al., 2016; Ogiwara et al., 2009) and autism spectrum disorder (ASD; Ben-Shalom et al., 2017). Efforts over the last two decades have led to the understanding that SCN2A mutations are the second-most common genetic contributor to EIMFS and ASD, after the potassium channel KCNT1 (Howell et al., 2015) and the fragile mental retardation protein FMRP (Ben-Shalom et al., 2017), respectively.
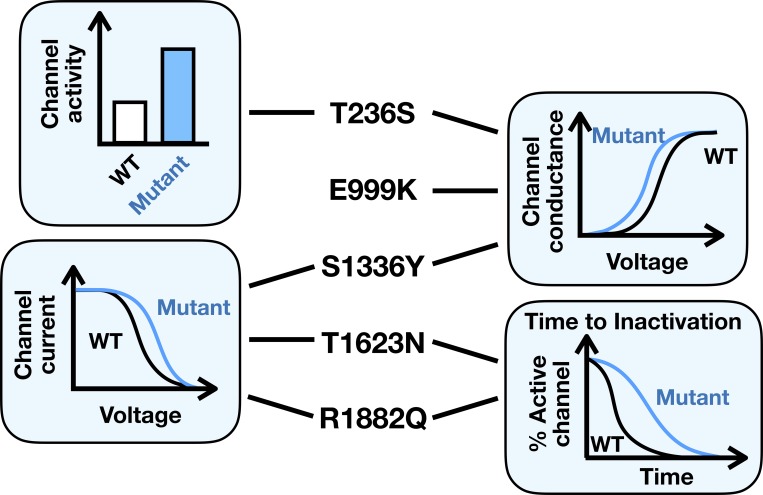
Thompson et al. (2020) examined the biophysical and cell functional ramifications of five mutants identified in patients with OS (Nakamura et al., 2013) or EIMFS (Howell et al., 2015), two of the most severe forms of EOEE. The mutants were expressed in the tsA201 heterologous expression system along with the β1 (SCN1B) and β2 (SCN2B) regulatory subunits, and channel electrophysiology studied using the whole-cell voltage clamp technique. The five mutants, which do not cluster to any distinct regions on the protein, conferred different changes to channel function (Fig. 1). The T236S mutant had increased sodium current without altering single channel conductance. Sodium channels are voltage-dependent in their activation, and T236S, E999K, and S1336Y mutants showed a heightened voltage sensitivity. Following activation, sodium channels enter into a state of inactivation, quieting channel activity for a time. This property is also voltage-dependent, and the mutants S1336Y, T1623N, and R1882Q required more depolarized membrane voltage before inactivating. Channel inactivation itself has a time-dependent component, and T1623N and R1882Q took longer to enter into the fast-inactivated state. When considered as a whole, these effects resulted in hyperactive channels. Computational modeling in a modified model of an immature neuron confirmed that these effects are likely to induce cell hyperexcitability.
Figure 1.
A pictorial summary of Thompson et al,’s work showing the types of gain-of-function changes the authors found for each of the five NaV1.2 mutant channels.
Similar changes have been reported by others examining different SCN2A mutations (for the most recent review, see Ben-Shalom et al., 2017). Thompson et al.’s findings also fit the paradigm that gain-of-function mutations are more likely to be associated with severe epilepsy while this holds true for loss-of-function mutations affecting those with less-severe epilepsy (Thompson et al., 2020; Begemann et al., 2019). Where Thompson et al.’s contribution is original, however, is that they investigated these five human mutations in the two splice isoforms that switch in abundance going from neonate to early childhood (Thompson et al., 2020). The two isoforms arise developmentally from the mutually exclusive insertion of exon 5: exon 5N designates the dominant neonatal form and exon 5A designates the post-neonatal adult form. These exons differ only in one amino acid at the 209 position: the neonatal isoform bears an asparagine (N), while the adult form incorporates the charged aspartic acid (D; Kasai et al., 2001). In mice, half the neonatal isoform is replaced by the adult form by P9, and from P15 on only about ∼10% of the neonatal isoform remains (Gazina et al., 2010). Functionally, the adult isoform of the channel is more active than the neonatal one, and a model neuron containing the adult channel is hyperactive compared with the neonatal model neuron (Xu et al., 2007). A few other studies have considered both isoforms in studying the effects of other SCN2A mutations (Xu et al., 2007; Liao et al., 2010b; Liao et al., 2010a), but no one has studied the ramifications of these particular mutations (T236S, E999K, S1336Y, T1623N, and R1882Q) in both the neonatal and adult isoforms.
Thompson et al. (2020) observed that some effects of the mutations were only manifest in the neonatal isoform. The twofold increase in sodium current of the neonatal T236S mutant compared with its wild type was no longer observed when the same comparison was made in the adult isoform. Likewise, only in the neonatal form did the mutant channels T236S, E999K, and S1336Y exhibit a heightened voltage-activation sensitivity. These findings imply that the brain of an infant harboring any of these mutations may experience hyperexcitability early in the infant’s life, coinciding with the clinical observation of seizures in the first days of life in these patients (Nakamura et al., 2013; Howell et al., 2015).
It feels intuitive that one should reproduce the neonatal milieu, including the neonatal specific isoform of a channel, when studying the effects of mutations that are associated with early-onset epilepsies. But will the choice of isoform determine the mutational effects?
Let us start with the null hypothesis that mutant channels will behave alike whether it occurs in the neonatal or the adult isoform. We can start by taking a mutation that has been examined in both isoforms of the human channel, L1563V. This mutation, which occurs in the second transmembrane region of SCN2A channel domain IV, was one of the first to be identified in a patient with BFNIS (Heron et al., 2002). This mutation has been studied in both human isoforms with and without the regulatory subunits (Xu et al., 2007; Misra et al., 2008; Berecki et al., 2018; Begemann et al., 2019). Xu et al. (2007) first reported that inactivation of the channel occurred more slowly in the mutant neonatal channel and that a greater, or more depolarized, membrane potential input was required to inactivate it. The neonatal L1563V was also quicker to recover from its inactivated state compared with the neonatal wild type. The adult wild type already recovers more quickly from inactivation compared with the neonatal wild type as described above, and this isoform was resistant to these mutational effects. Mathematical modeling confirmed that a model neuron with the mutant neonatal channel was more excitable compared with the wild type neonatal channel. As per model neurons with the adult channels, the mutant neuron was only slightly more excitable than the wild type counterpart. In summary: like a child playing make believe as a grownup, the mutant neonatal channel mimics the adult wild type channel properties. And once in the adult channel isoform, the mutant channel ceases its mimicry.
It appears then that we should reject the null hypothesis. But it is more nuanced than that, as others have observed differences in an adult form of the mutant channel compared with the adult wild type channel. The adult L1563V mutant channels continue to show a more depolarized voltage-dependent inactivation compared with the adult wild type (Misra et al., 2008; Berecki et al., 2018; Begemann et al., 2019). A similar pattern has also been observed in the C-terminal R1882Q mutation found in EIMFS patients (Table 1). Whether expressed in the neonatal or adult isoforms, the R1882Q channel has a more depolarized voltage-dependent inactivation pattern. The mutant channel is also slower in entering into the fast-inactivated state irrespective of the isoform type (Berecki et al., 2018). Hence, it may be that some effects of the mutations observable in the neonatal isoform will persist into the adult isoform.
Table 1. Comparison of NaV1.2 L1563V and R1882Q electrophysiology studies.
| Disease | SCN2A α subunit | Experimental conditions | Channel electrophysiological properties | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Species | Isoform | Exogenous β1/β2 subunit | Cell type | Current density | V-dependent activation | V-dependent inactivation | Fast inactivation time constant τ | Persistent inward current | Recovery from inactivation | ||
| BFNIS | L1563V | Human | Neonatal | Neither | tsA201 | Unchanged | Unchanged | Depolarized | Greater | Unchanged | Quicker | Xu et al., 2007 |
| Adult | Neither | tsA201 | Unchanged | |||||||||
| Adult | β1 and β2 | tsA201 | Decreased | Unchanged | Depolarized | Greater | Unchanged | Quicker | Misra et al., 2008 | |||
| Adult | Neither | Chinese hamster ovarian cells | Unchanged | Depolarized | Depolarized | Like the adult WT | Unchanged | Quicker | Berecki et al., 2018 | |||
| Adult | β1 and β2 | HEK293T | Unchanged | Unchanged | Depolarized | Like the adult WT | Unchanged | Unchanged | Begemann et al., 2019 | |||
| EIMFS | R1882Q | Human | Adult | Neither | Chinese hamster ovarian cells | Increased | Hyperpolarized | Depolarized | Greater | Increased | Unchanged | Berecki et al., 2018 |
| Neonatal | β1 and β2 | tsA201 | Unchanged | Unchanged | Depolarized | Greater | Unchanged | Unchanged | Thompson et al., 2020 | |||
| Adult | β1 and β2 | tsA201 | Unchanged | Depolarized | Depolarized | Greater | Unchanged | Unchanged | ||||
Nevertheless, differences in experimental design (Table 1) and the small sample size reveal major limitations for the analysis at hand. The inclusion of the β1 and β2 regulatory subunits, or the cell type used may alter the effects of the mutation on channel electrophysiology, and the specific conditions vary among these studies. Interestingly, there are disease-causing mutations within the developmentally regulated exon 5. They occur immediately downstream of the neonatal/adult N209D and lead to the substitution of a charged aspartic acid: G211D (Kodera et al., 2013), N212D, and V213D (Nakamura et al., 2013). Given their proximity and specific substitution, any differential effects of these mutations in the neonatal versus adult isoforms may help further weigh in on the relevance of using either isoform in future studies of EOEE-related NaV1.2 mutations.
What do Thompson et al.’s findings mean when considered clinically? With more data points, these results could be translated into a roadmap for those clinicians advising a patient with a particular mutation on what the prognosis may be and what treatment regiments might be most favorable. Nevertheless, it remains unknown how mutations affecting the same gene could lead to a spectrum of diseases of varying severity. SCN2A mutations are found in those with severe as well as benign forms of epilepsies, a conundrum also observed among those who have mutations in the sodium-activated potassium channel Slack (KCNT1; Kim and Kaczmarek, 2014). KCNT1 and SCN2A patients do share similarities in that their cognitive development and function are affected, and mutations in either are the most likely genetic alterations to be found in patients with ASD or the fragile x syndrome (Kim and Kaczmarek, 2014; Ben-Shalom et al., 2017). It may be that there is a third common protein regulator or neuronal modulator at the systems level that ultimately determines the severity of disease, though this remains just a speculation. Uncovering the answer will require further mechanistic insights into individual channel mutations, more refined and complex mathematical modeling, and animal models of these disease types. This feels immense in scope, but just 20 years ago such vision was inconceivable. And as the field advances, I hope that we will all benefit from its collective wisdom.
Acknowledgments
Joseph A. Mindell served as editor.
This work was supported by the American Heart Association grant 18POST33960157 awarded to G.K. Muller.
The author declares no competing financial interests.
References
- Begemann A., Acuña M.A., Zweier M., Vincent M., Steindl K., Bachmann-Gagescu R., Hackenberg A., Abela L., Plecko B., Kroell-Seger J., et al. . 2019. Further corroboration of distinct functional features in SCN2A variants causing intellectual disability or epileptic phenotypes. Mol. Med. 25:6 10.1186/s10020-019-0073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shalom R., Keeshen C.M., Berrios K.N., An J.Y., Sanders S.J., and Bender K.J.. 2017. Opposing Effects on NaV1.2 Function Underlie Differences Between SCN2A Variants Observed in Individuals With Autism Spectrum Disorder or Infantile Seizures. Biol. Psychiatry. 82:224–232. 10.1016/j.biopsych.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berecki G., Howell K.B., Deerasooriya Y.H., Cilio M.R., Oliva M.K., Kaplan D., Scheffer I.E., Berkovic S.F., and Petrou S.. 2018. Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy. Proc. Natl. Acad. Sci. USA. 115:E5516–E5525. 10.1073/pnas.1800077115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield C.S., Camfield P.R., Gordon K., Wirrell E., and Dooley J.M.. 1996. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 37:19–23. 10.1111/j.1528-1157.1996.tb00506.x [DOI] [PubMed] [Google Scholar]
- Engel J. Jr. International League Against Epilepsy (ILAE) . 2001. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 42:796–803. 10.1046/j.1528-1157.2001.10401.x [DOI] [PubMed] [Google Scholar]
- Gazina E.V., Richards K.L., Mokhtar M.B., Thomas E.A., Reid C.A., and Petrou S.. 2010. Differential expression of exon 5 splice variants of sodium channel alpha subunit mRNAs in the developing mouse brain. Neuroscience. 166:195–200. 10.1016/j.neuroscience.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Heron S.E., Crossland K.M., Andermann E., Phillips H.A., Hall A.J., Bleasel A., Shevell M., Mercho S., Seni M.H., Guiot M.C., et al. . 2002. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet. 360:851–852. 10.1016/S0140-6736(02)09968-3 [DOI] [PubMed] [Google Scholar]
- Howell K.B., McMahon J.M., Carvill G.L., Tambunan D., Mackay M.T., Rodriguez-Casero V., Webster R., Clark D., Freeman J.L., Calvert S., et al. . 2015. SCN2A encephalopathy: A major cause of epilepsy of infancy with migrating focal seizures. Neurology. 85:958–966. 10.1212/WNL.0000000000001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai N., Fukushima K., Ueki Y., Prasad S., Nosakowski J., Sugata K., Sugata A., Nishizaki K., Meyer N.C., and Smith R.J.. 2001. Genomic structures of SCN2A and SCN3A - candidate genes for deafness at the DFNA16 locus. Gene. 264:113–122. 10.1016/S0378-1119(00)00594-1 [DOI] [PubMed] [Google Scholar]
- Kim G.E., and Kaczmarek L.K.. 2014. Emerging role of the KCNT1 Slack channel in intellectual disability. Front. Cell. Neurosci. 8:209 10.3389/fncel.2014.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera H., Kato M., Nord A.S., Walsh T., Lee M., Yamanaka G., Tohyama J., Nakamura K., Nakagawa E., Ikeda T., et al. . 2013. Targeted capture and sequencing for detection of mutations causing early onset epileptic encephalopathy. Epilepsia. 54:1262–1269. 10.1111/epi.12203 [DOI] [PubMed] [Google Scholar]
- Lauxmann S., Boutry-Kryza N., Rivier C., Mueller S., Hedrich U.B., Maljevic S., Szepetowski P., Lerche H., and Lesca G.. 2013. An SCN2A mutation in a family with infantile seizures from Madagascar reveals an increased subthreshold Na(+) current. Epilepsia. 54:e117–e121. 10.1111/epi.12241 [DOI] [PubMed] [Google Scholar]
- Liao Y., Anttonen A.K., Liukkonen E., Gaily E., Maljevic S., Schubert S., Bellan-Koch A., Petrou S., Ahonen V.E., Lerche H., and Lehesjoki A.E.. 2010a SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 75:1454–1458. 10.1212/WNL.0b013e3181f8812e [DOI] [PubMed] [Google Scholar]
- Liao Y., Deprez L., Maljevic S., Pitsch J., Claes L., Hristova D., Jordanova A., Ala-Mello S., Bellan-Koch A., Blazevic D., et al. . 2010b Molecular correlates of age-dependent seizures in an inherited neonatal-infantile epilepsy. Brain. 133:1403–1414. 10.1093/brain/awq057 [DOI] [PubMed] [Google Scholar]
- Misra S.N., Kahlig K.M., and George A.L. Jr. 2008. Impaired NaV1.2 function and reduced cell surface expression in benign familial neonatal-infantile seizures. Epilepsia. 49:1535–1545. 10.1111/j.1528-1167.2008.01619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Kato M., Osaka H., Yamashita S., Nakagawa E., Haginoya K., Tohyama J., Okuda M., Wada T., Shimakawa S., et al. . 2013. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 81:992–998. 10.1212/WNL.0b013e3182a43e57 [DOI] [PubMed] [Google Scholar]
- Ogiwara I., Ito K., Sawaishi Y., Osaka H., Mazaki E., Inoue I., Montal M., Hashikawa T., Shike T., Fujiwara T., et al. . 2009. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology. 73:1046–1053. 10.1212/WNL.0b013e3181b9cebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalmani P., Rusconi R., Armatura E., Zara F., Avanzini G., Franceschetti S., and Mantegazza M.. 2006. Effects in neocortical neurons of mutations of the Na(v)1.2 Na+ channel causing benign familial neonatal-infantile seizures. J. Neurosci. 26:10100–10109. 10.1523/JNEUROSCI.2476-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., Hahn A., Bast T., Müller S., Löffler H., Maljevic S., Gaily E., Prehl I., Biskup S., Joensuu T., et al. . 2016. Mutations in the sodium channel gene SCN2A cause neonatal epilepsy with late-onset episodic ataxia. J. Neurol. 263:334–343. 10.1007/s00415-015-7984-0 [DOI] [PubMed] [Google Scholar]
- Su D.J., Lu J.F., Lin L.J., Liang J.S., and Hung K.L.. 2018. SCN2A mutation in an infant presenting with migrating focal seizures and infantile spasm responsive to a ketogenic diet. Brain Dev. 40:724–727. 10.1016/j.braindev.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Sugawara T., Tsurubuchi Y., Agarwala K.L., Ito M., Fukuma G., Mazaki-Miyazaki E., Nagafuji H., Noda M., Imoto K., Wada K., et al. . 2001. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc. Natl. Acad. Sci. USA. 98:6384–6389. 10.1073/pnas.111065098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.H., Ben-Shalom R., Bender K.J., and George A.L.. 2020. Alternative splicing potentiates dysfunction of early-onset epileptic encephalopathy SCN2A variants. J. Gen. Physiol. 152:e201912442 10.1085/jgp.201912442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkdogan D., Thomas G., and Demirel B.. 2019. Ketogenic diet as a successful early treatment modality for SCN2A mutation. Brain Dev. 41:389–391. 10.1016/j.braindev.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Wolff M., Johannesen K.M., Hedrich U.B.S., Masnada S., Rubboli G., Gardella E., Lesca G., Ville D., Milh M., Villard L., et al. . 2017. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain. 140:1316–1336. 10.1093/brain/awx054 [DOI] [PubMed] [Google Scholar]
- Xu R., Thomas E.A., Jenkins M., Gazina E.V., Chiu C., Heron S.E., Mulley J.C., Scheffer I.E., Berkovic S.F., and Petrou S.. 2007. A childhood epilepsy mutation reveals a role for developmentally regulated splicing of a sodium channel. Mol. Cell. Neurosci. 35:292–301. 10.1016/j.mcn.2007.03.003 [DOI] [PubMed] [Google Scholar]