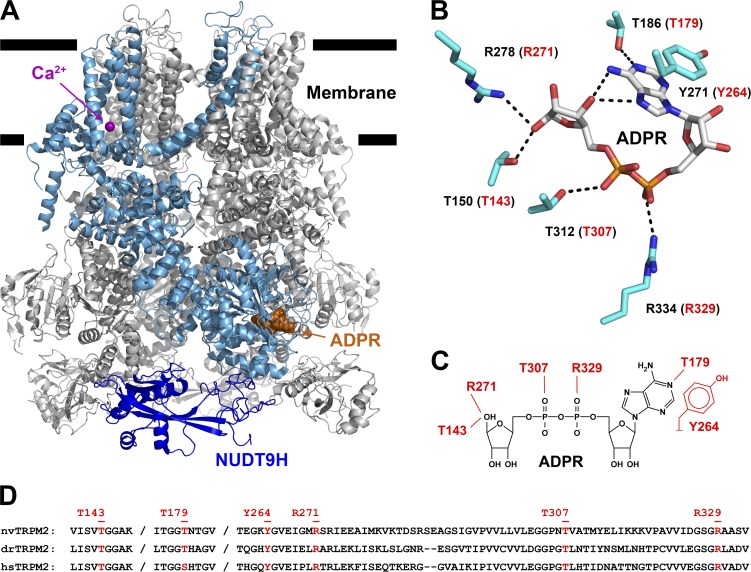
Figure 3.
Amino acid side chains interacting with ADPR bound in the MHR1/2 domain. (A) Cartoon representation of drTRPM2 structure solved in the presence of ADPR+Ca2+ (PDB accession no. 6DRJ). One subunit is highlighted in light blue, except for the NUDT9-H domain, which is shown in dark blue. ADPR is represented in orange spacefill and Ca2+ as purple sphere. (B) Stick representation of ADPR bound in the MHR1/2 domain of drTRPM2 and six amino acid side chains (black labels) in contact with the nucleotide. Dotted black lines represent polar contacts identified by PyMOL. Red labels in parentheses refer to corresponding nvTRPM2 residues. (C) Schematic representation of ADPR and expected interacting residues in the MHR1/2 domain of nvTRPM2. (D) Sequence alignment of nvTRPM2, drTRPM2, and hsTRPM2 peptide segments involved in ADPR coordination in the N-terminal site. Residues targeted for mutagenesis are highlighted in red, with nvTRPM2 sequence numbering shown on top.