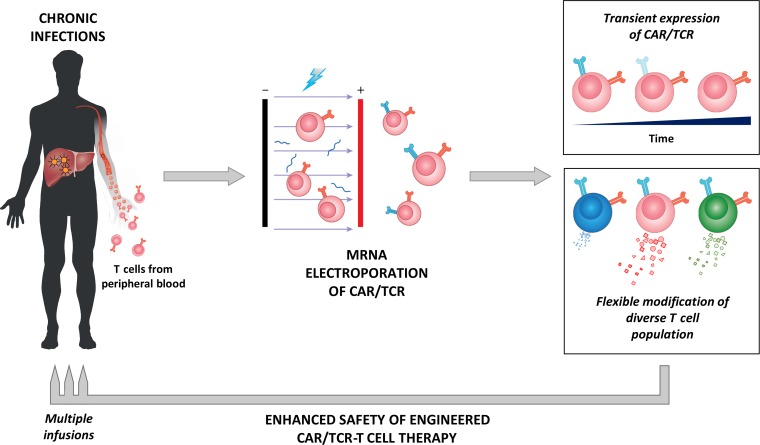
Figure 1.
CAR/TCR-T cells engineered through mRNA electroporation confer enhanced safety features suited for their deployment in patients with chronic viral diseases. The labile nature of mRNA results in a transient expression of transgenic CAR/TCR that limits the functional lifespan of the engineered T cells to ~3–5 days. This allows for a conservative dose escalation clinical trial design to closely monitor for potential toxicities. The flexibility of the technique to modify even non-activated T cells resulting in CAR/TCR-T cells with reduced cytolytic capacity, while maintaining antiviral effects, can further enhance the safety of the approach in viral infected patients.