Figure 7. LPS sensing of MSCs shapes the function of neutrophils at the wound site.
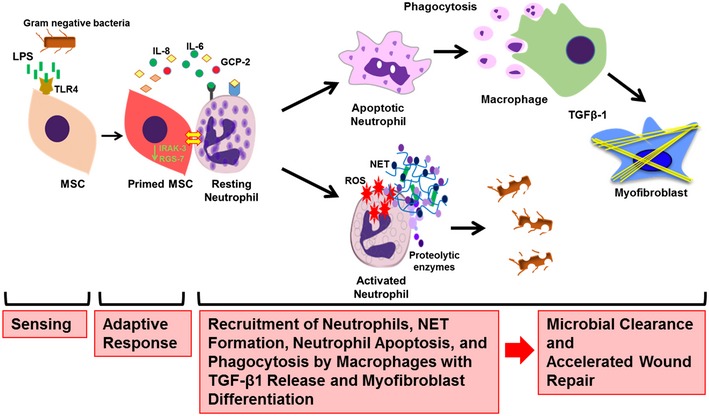
Graphical summary, depicting the molecules involved in sensing, signaling, and raising an adaptive response in MSCs. MSCs sense bacterial intruders as modeled by the key molecule LPS, a widely distributed PAMP and wall component of Gram‐negative bacteria. LPS is sensed via the TLR4 receptor, and the signal is relayed via MyD88 to downstream effectors which, in consequence, shape the adaptive MSC response. This response is enforced by fundamental transcriptomic reprogramming which is responsible for the release of factors critical for neutrophil and macrophage recruitment to the wound site. The adaptive response of LPS‐primed MSCs depicts a bifurcation with the activation of neutrophils with enhanced microbicidal NET formation and ROS release to directly counteract invading bacteria. In addition, in a second line of tissue protection and repair, apoptotic neutrophils are phagocytosed by macrophages. Neutrophil engulfment constitutes a strong signal for macrophages to release TGFβ‐1 which subsequently enhances differentiation of myofibroblasts and accelerates wound contraction and thus wound closure. Acceleration of wound closure together with enhanced NET formation and ROS release effectively counteracts microbial invasion. These data may stimulate new avenues to refine MSC‐based therapies for difficult‐to‐heal wounds and/or infected wounds.
