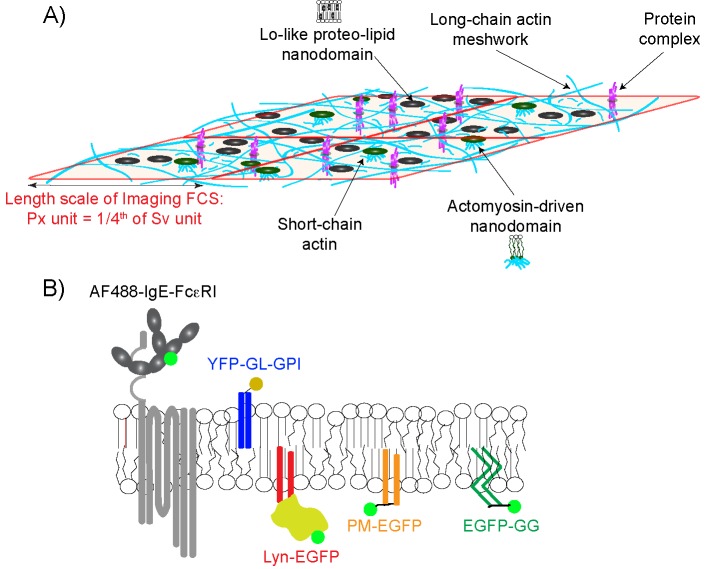
FIGURE 1:
A composite of plasma membrane organization may be determined by monitoring the diffusion of structurally distinct probes. (A) The plasma membrane is organized at different length scales in a hierarchical scheme: a relatively static actin meshwork (cyan long chains); dynamic Lo-like proteolipid nanodomains (black circles) with variable physical properties within and across leaflets; transmembrane ordered lipids mediated by dynamic, myosin-driven assembly of short actin chains (green circles connected to short cyan chains); and stable or dynamic protein complexes. We note that Lo-like proteolipid nanodomains and protein complexes are much smaller than the dimensions of the actin meshwork and are not drawn to scale here. Interaction of a probe with these organizational features retards its diffusion, depending on that probe’s physicochemical properties. ImFCS measures diffusion coefficients (D) at the resolution of a Px unit (320 × 320 nm2), which has dimensions considerably larger than the actin meshwork; parameters τ0 and 1/Deff = Slope are measured in Sv units that comprise a square of 16 Px units. These measurements are illustrated in Figure 2. (B) Fluorescent lipid, lipid-anchored, and transmembrane (TM) probes are evaluated by ImFCS. AF488-IgE-FcεRI: transmembrane with seven TM regions; YFP-GL-GPI: outer-leaflet lipid probe with saturated acyl chain anchor and an extracellular consensus glycosylation site; Lyn-EGFP: inner leaflet probe with saturated acyl chain anchors and additional cytosolic protein modules; PM-EGFP: inner leaflet lipid probe with same saturated acyl chain anchors of Lyn-EGFP; EGFP-GG: inner leaflet lipid probe with unsaturated acyl chain anchors and membrane-proximal basic sequence. Previous studies showed that lipid probes YFP-GL-GPI, PM-EGFP dynamically partition into Lo-like proteolipid nanodomains, whereas EGFP-GG partitions less favorably into these nanodomains, preferring Ld-like regions.