Figure 6. Histopathologic changes in the lungs of immunocompetent and immunosuppressed mice inoculated with influenza BR/08 virus and treated with oseltamivir.
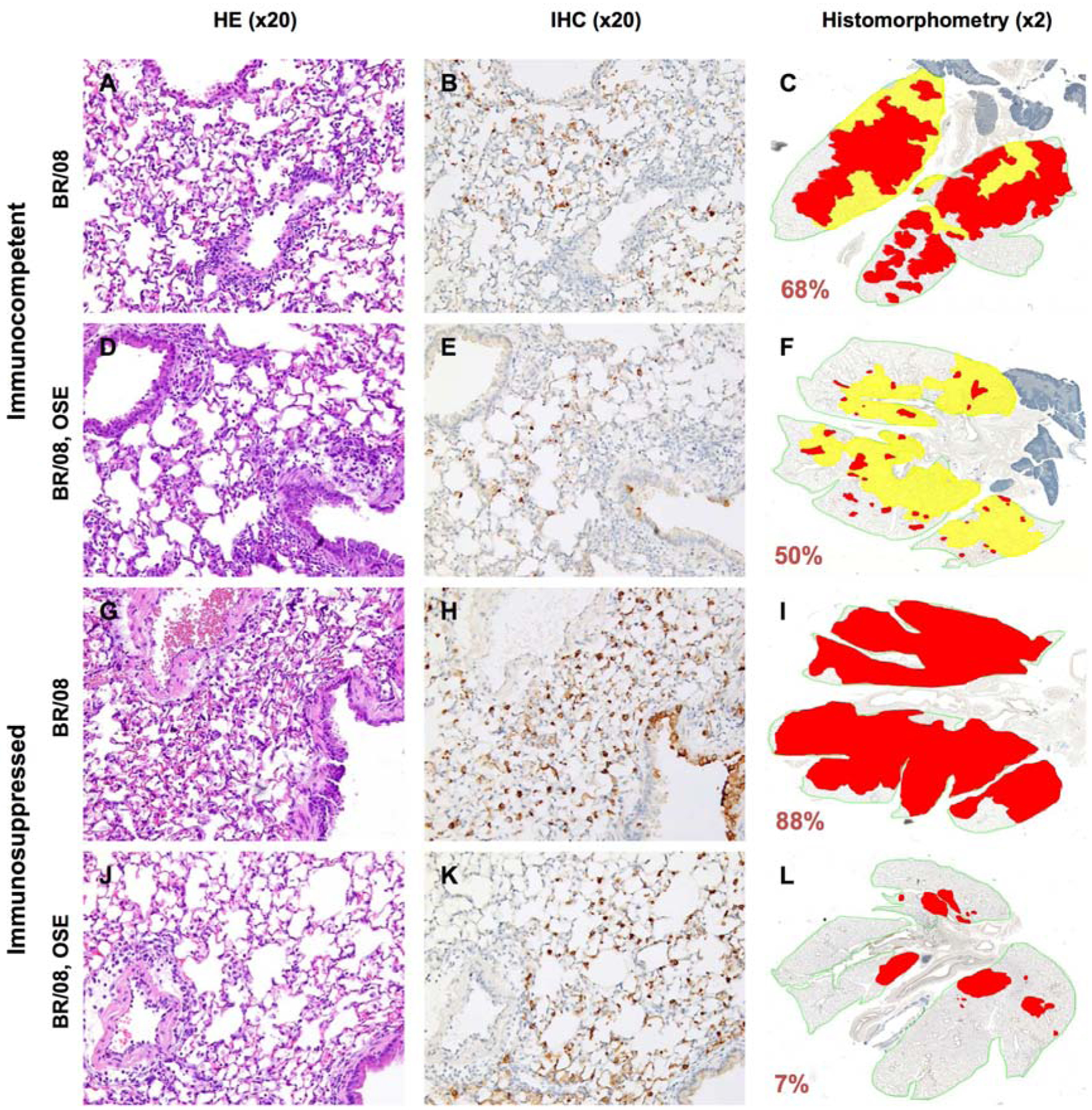
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with a 5 MLD50 dose of BR/08 virus. The NAI oseltamivir was administered by oral gavage at a dose of 20 mg/kg/day (0.1 mL/mouse) twice daily for 8 (immunocompetent mice) or 16 (immunosuppressed mice) days. Control animals received sterile water for 8 days. Pulmonary lesions were evaluated at 6 dpi (n = 2/group). Mouse lungs were fixed in 10% neutral-buffered formalin and stained with HE (A, D, G, and J), subjected to IHC staining with anti–influenza B antiserum (B, E, H, and K), or analyzed by histomorphometry (C, F, I, and L). Representative images for each treatment group are shown (magnification: ×20 [A, B, D, E, G, H, J, and K] or ×2 [C, F, I, and L]). The total lung areas examined are outlined in green; areas of active infection with antigen-positive cells are shown in red, and areas of inactive infection with lesions but negligible antigen are shown in yellow. The percentage of the total lung area represented by the lesions is indicated for each image.
