Abstract
Background
This is an updated version of the original Cochrane review published in Issue 4, 2012. Myofascial pain syndrome (MPS) is a regional muscular pain syndrome characterised by the presence of trigger points, which are painful points in one or more muscles. The pain can be felt at the site where the trigger point is located or it can be felt away from that place when the muscle is pressed (referred pain). Botulinum toxin is a protein produced by the bacterium Clostridium botulinum and is a potent neurotoxin that eventually inhibits muscle contractions. It is capable of selectively weakening painful muscles and interrupting the pain cycle.
Objectives
To assess the effectiveness and safety of botulinum toxin A (BTXA) in the treatment of myofascial pain syndrome (MPS), excluding MPS in neck and head muscles.
Search methods
This is an updated version of the original Cochrane review published in Issue 4, 2012. The search strategy for the update was the same as in the original review and we searched CENTRAL in The Cochrane Library (2013, Issue 11 of 12), MEDLINE (Ovid) (2012 to 29 November 2013) and EMBASE (Ovid) (2012 to 27 November 2013). The search strategy was composed of terms for myofascial pain and botulinum toxin. For the original review, we also searched the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group Specialised Register until December 2011, PubMed (from 1966 to 2011) and LILACS (from 1982 to 2011). There was no language restriction.
Selection criteria
We included randomised controlled trials (RCTs) involving botulinum toxin for treating participants with MPS. We excluded studies with MPS of the neck and head from this review as they have already been assessed in existing systematic reviews. We considered a diagnosis of MPS to be based on the identification of trigger points in the taut band through palpation of sensitive nodules, local twitch response and specific patterns of referred pain associated with each trigger point.
Data collection and analysis
Two review authors independently screened identified studies, extracted data, assessed trial quality and analysed results using the Cochrane PaPaS Review Group criteria.
Main results
Four studies with a total of 233 participants, comparing BTXA with placebo, met the inclusion criteria. In one study with 145 participants, significant improvement rates of pain intensity scores and duration of daily pain were demonstrated when comparing BTXA with placebo. The three other studies showed that there was no statistically significant difference between BTXA and placebo in pain intensity.
Authors' conclusions
Since the first publication of this review, no new studies were found. There is inconclusive evidence to support the use of botulinum toxin in the treatment of MPS based on data from four studies with a total of 233 participants, which we considered were of sufficient quality to be included in this review. Meta‐analyses were not possible due to the heterogeneity between studies. We suggest that in future studies the same methodology to assess pain, a standardised dose of treatment, follow‐up of at least four months (to observe the maximum and minimum curve of the drug effect) and appropriate data presentation should be used. More high‐quality RCTs of botulinum toxin for treating MPS need to be conducted before firm conclusions on its effectiveness and safety can be drawn.
Plain language summary
Botulinum toxin injectable drug for myofascial pain syndrome (a painful condition that could affect any muscle in the body) in adults
Myofascial pain syndrome (MPS) is a regional muscular pain syndrome characterised by the presence of trigger points (painful points) in one or more muscles. The pain can be at the trigger point site or it can be felt away from that point when the muscle is pressed, which is called referred pain. Botulinum toxin is a potent chemical that blocks the communication between nerves and muscles and inhibits muscle contractions in the muscles where the trigger points are located, which may result in pain relief when it is injected into the area. The purpose of this review was to assess how effective botulinum toxin is at reducing pain in patients with MPS. We identified four studies, with 233 participants, comparing botulinum toxin A with placebo (control group). There was inconclusive evidence to support the use of botulinum toxin in the treatment of MPS. More high‐quality randomised controlled trials of botulinum toxin for treating MPS need to be conducted before firm conclusions on its effectiveness and safety can be drawn.
Background
This is an update of a previously published review in the Cochrane Database of Systematic Reviews (2012, Issue 4) on 'Botulinum toxin for myofascial pain syndromes in adults'.
Description of the condition
Myofascial pain syndrome (MPS) is characterised by acute or chronic regional muscle pain associated with single or multiple restricted, painful regions (trigger points) within taut muscle bands. It is also associated with stiffness and local twitch response when stimulated by digital pressure or during located needling, generating local or referred pain (Ernberg 2002; Gerwin 1995; Guststein 1938; Simons 1976; Simons 1999; Sola 1955).
Individuals who have regional pain present with a high prevalence of MPS. Studies carried out in pain management centres and clinics of different specialties have shown a prevalence ranging from 30% to 93% (mean 6 in 10) in participants complaining of regionalised pain (Fishbain 1986; Fricton 1985; Gerwin 1995; Han 1997; Schiffman 1990; Skootsky 1989), in 30 to 50 year‐olds (Simons 1999) and in women (Han 1997; Sola 1955).
The trapezius, levator scapula, infraspinatus and scalenes muscles of the upper body are the areas most frequently affected by MPS (Sola 1955). A number of causal factors have been suggested for MPS such as acute physical overload, deep pain impulse, emotional tension, postural habits, fatigue, hypovitaminosis, infections, physical inactivity, poor physical conditioning, repetitive musculoskeletal microtraumas and trauma (Edwards 2005; Fricton 1985; Fricton 1994; Laskin 1969; Simons 1976; Simons 1999).
The diagnosis of MPS is based on the identification of trigger points in the taut band through palpation of sensitive nodules, local twitch response and specific patterns of pain referral associated with each trigger point (Fricton 1985; Simons 1999).
MPS treatment can be multidimensional and consists of trigger point inactivation, which breaks the vicious cycle of pain‐spasm‐pain (Simons 1999). It also includes reassurance (patient education, self care and behaviour therapy), physiotherapy (ultrasound, mega pulse, low‐level laser therapy, massage, transcutaneous electrical nerve stimulation (TENS), application of warm compresses, exercises (such as post‐isometric relaxation and stretching, biofeedback, cold spray and stretch techniques), acupuncture, dry needling, injections of anaesthetic, drug therapy and combined treatments (Baldry 2002; Bron 2007; Dundar 2007; Esenyel 2000; Flor 1993; Han 1997; Hanten 2000; Hong 1994; Kam 2002; McMillan 1997; Roth 1998; Simons 1999; Solberg 1986; Srbely 2007; Talaat 1986). Drugs used to treat MPS includes analgesics, non‐steroidal anti‐inflammatory drugs (NSAIDS), muscle relaxants and tricyclic antidepressants (Borg‐Stein 2006; Pettengill 1997; Singer 1997).
Description of the intervention
Botulinum toxin, which is a potent neurotoxin synthesised by the bacterium Clostridium botulinum, blocks acetylcholine release into the neuromuscular junction, leading to reduced muscular contraction (Sellin 1981). A typical duration of effect is three to four months (Borodic 2001). Currently, only botulinum toxin serotypes A and B (BTXA and BTXB) are approved by the US Food and Drug Administration (FDA) for diseases with a neurological component (Lew 2002).
How the intervention might work
It is presumed that botulinum toxin breaks the spasm and pain cycle by blocking the release of acetylcholine, with a duration of effect of a few months, giving the patient an opportunity for traditional conservative measures to have a greater beneficial impact (Borodic 2001; Howard 2002).
BTXA has been used effectively in the treatment of cervical dystonia, spasticity and hyperkinetic facial lines, and is emerging as a potential treatment for phantom limb pain, complex regional pain syndrome, myofascial pain and headache (Argoff 2002; Borodic 2001; Brashear 1999; Kern 2004; Royal 2001).
Why it is important to do this review
There is available evidence from different study designs, including a previous systematic review and non‐randomised studies (Pereda 2006; Royal 2001), suggesting that botulinum toxin has the potential to be used in participants being treated for MPS. On the other hand, a systematic review with meta‐analysis including 139 participants with neck disorders (Peloso 2007) suggested that there was no significant improvement in participants taking BTXA versus those taking placebo in the treatment of MPS. A systematic review of randomised controlled trials (RCTs) including other muscles from different parts of the body and higher numbers of participants is necessary to clarify this issue.
Objectives
To assess the effectiveness and safety of botulinum toxin A (BTXA) in the treatment of myofascial pain syndrome (MPS), excluding MPS in neck and head muscles.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs involving botulinum toxin for treating participants with MPS, with at least two comparison arms. We excluded studies that included participants with head and neck myofascial pain from this review as two systematic reviews by Peloso 2007 and Langevin 2011 have already been published in this area.
Types of participants
Both male and female participants aged 18 years or above with a clinical diagnosis of MPS, regardless of race, social and economic status, profession or residential location. We considered a diagnosis of MPS to be based on the identification of trigger points in the taut band through palpation of sensitive nodules, local twitch response and specific patterns of referred pain associated with each trigger point. We chose not to include participants for whom botulinum toxin was contraindicated, as follows:
history of hypersensitivity to any component of the product;
disturbances of muscular activity in concomitant treatment with antibiotics aminoglycoside or spectinomycin;
any bleeding disorders;
treatment with anticoagulants;
any reason to avoid injection of intramuscular agents;
those that were pregnant or breastfeeding.
Types of interventions
Intervention group: botulinum toxin A, irrespective of dose
Control group: placebo, alternative drug
Co‐interventions were accepted if applied in all comparison groups
Types of outcome measures
Primary outcomes
Intensity, frequency and duration of pain, pain relief, and pressure pain tolerance recorded using validated visual analogue scales (VAS) or categorical scales
Secondary outcomes
Disability
Quality of life (QoL) measured by Nottingham Health Profile (NHP) and Short Form‐36 (SF‐36)
Improving the range of motion
Adverse events
Search methods for identification of studies
This search was run for the original review on 15 December 2011 and subsequent searches were run on 3 December 2013.
Electronic searches
For this update we searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 11 of 12);
MEDLINE (Ovid);
EMBASE (Ovid).
To identify studies for the original review, we searched the following databases:
the Cochrane Pain, Palliative and Supportive Care Review Group Specialised Register (until December 2011);
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (until Issue 4, December 2011);
PubMed (from 1966 to 2011);
EMBASE (from 1980 to 2011);
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS) (from 1982 to 2011).
The search of the Cochrane Pain, Palliative and Supportive Care Review Group Specialised Register was not updated in 2013 because the Register is no longer updated, and MEDLINE was searched instead of PubMed.
The search strategy was composed of terms for myofascial pain and botulinum toxin. As we searched with both subject headings and free‐text words, we expected to identify all available studies on myofascial pain and botulinum toxin. We used terms and their synonyms for myofascial pain and botulinum toxin to compose a sensitive search strategy (please see Appendix 1 for the search strategies). There was no language restriction.
Searching other resources
We checked reference lists of the included studies manually to identify any additional studies and contacted specialists in the field and authors of the included studies for unpublished data.
Data collection and analysis
Selection of studies
Two review authors (AS and ES) independently screened the studies identified by the literature search, extracted the data, assessed study quality and analysed the results. Two review authors (AS and ES) performed the selection of the titles and the evaluation of methodological quality of the RCTs. We measured inter‐observer concordance between AS and ES, using the Kappa coefficient, for titles and abstracts with potential to be included.
Data extraction and management
Two review authors (AS and ES) extracted data independently. We resolved discrepancies in the results by discussion. We initially used a standard form to extract the following information: characteristics of the study (design, methods of randomisation); participants; interventions; outcomes (types of outcome measures, timing of outcome pain scores such as visual analogue scale (VAS) or total pain scale, adverse events). We based the form on the recommendations made by the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group and used it as a first step in the process of data extraction.
Assessment of risk of bias in included studies
We evaluated the methodological quality of the selected studies according the Cochrane 'risk of bias' tool.
Random sequence generation
Low: random number table, computer random number generator
Unclear: method not clearly stated
High: no random process (e.g. hospital or clinic record number, date of admission)
Allocation concealment
Low: telephone or central randomisation, consecutively numbered sealed opaque envelopes
Unclear: method not clearly stated
High: open list
Blinding of outcome assessment
Study participants
Low: patient was unaware of the assigned treatment Unclear: blinding not reported and could not be verified by contacting the investigators High: participants were aware of the assigned treatment (open‐label studies)
Outcome assessors
Low: assessors were unaware of the assigned treatment when collecting outcome measures Unclear: blinding of assessor was not reported and could not be verified by contacting the investigators High: assessors were aware of the assigned treatment when collecting outcome measures
Incomplete outcome data
Low: less than or equal to 10% for both groups Unclear: used 'last observation carried forward' analysis High: used 'completer' analysis
Size of study
Low: > 200 participants per treatment arm
Unclear: 50 to 199 participants per treatment arm
High: < 50 participants per treatment arm
Measures of treatment effect
In the original review, for dichotomous data we used risk ratio (RR), percentage of events and 95% confidence interval (CI) as the effect measure and for continuous data we used mean difference (MD) or standardised mean difference (SMD) with their respective 95% CI as appropriate (Unnebrink 2001). For this update, we did not perform any meta‐analysis, and removed the results of the original analyses in line with current Cochrane methodological standards.
Unit of analysis issues
For the original review, we based the unit of analysis on the individual participant (unit randomised for interventions to be compared), i.e. the number of observations in the analysis should match the number of individuals randomised. For this update, we did not perform any meta‐analysis, and removed the results of the original analyses in line with current Cochrane methodological standards.
Dealing with missing data
For missing or unavailable data, we planned to contact the study authors for additional information. In the case of no response, irrespective of the type of data, we planned to report dropout rates in the 'Characteristics of included studies' tables of the review (Higgins 2008).
Assessment of heterogeneity
For the original review, we quantified inconsistency among the pooled estimates using the I2 statistic (Higgins 2003; Higgins 2008). We used a fixed‐effect model in the absence of substantial heterogeneity (I2 < 50%), otherwise we used a random‐effects model (I2 > 50%) (Higgins 2008). For this update, we did not perform any meta‐analysis, and removed the results of the original analyses in line with current Cochrane methodological standards.
Assessment of reporting biases
In future updates, if a sufficient number of studies are available, we plan to assess publication bias by drawing a funnel plot (trial effect versus trial size). However, this tool may be misleading (Tang 2000; Thornton 2000) and we will not place undue emphasis upon any results it provides.
Data synthesis
Methods of synthesising the studies will depend on quality, design and heterogeneity. We planned to explore both clinical and statistical heterogeneity. In the absence of clinical and statistical heterogeneity (I2 < 50%) we planned to apply a fixed‐effect model to pool the data. In the presence of statistical heterogeneity (I2 > 50%) we planned to apply a random‐effects model for meta‐analysis. Where synthesis was inappropriate we planned to present a narrative overview.
Subgroup analysis and investigation of heterogeneity
For the original review we performed subgroup analysis to explore:
the influence of the type, regimen and dosage of botulinum toxin;
co‐interventions (e.g. rehabilitation programmes);
muscle group, age range and gender.
We expected that a diversity of follow‐up times would be found across included studies (for example one to four months), which could also motivate subgroup analysis. For this update, we did not perform any meta‐analysis, and removed the results of the original analyses in line with current Cochrane methodological standards.
Sensitivity analysis
We carried out no sensitivity analysis as no data pooling was possible. In future updates, in anticipation of more studies, we will perform sensitivity analysis to explore the robustness of the results. The following factors will motivate the sensitivity analysis:
quality of allocation concealment (adequate, unclear or inadequate);
double‐blind method (adequate, unclear, inadequate or not performed);
unpublished studies;
study design (parallel versus cross‐over).
Results
Description of studies
Results of the search
The search strategy for the original review (run in December 2011) retrieved 401 records: PaPaS Specialised Register with 20 references; The Cochrane Library with 20 references (CENTRAL, Cochrane Reviews and DARE for other reviews); MEDLINE (Ovid) with 165 references; EMBASE with 174 references and LILACS with 22 references. We also scrutinised the bibliographical references of these papers for further potentially eligible studies and found no additional references. The updated search, performed in December 2013, identified a further 69 studies. No additional studies were eligible for inclusion in this update (see Figure 1).
1.
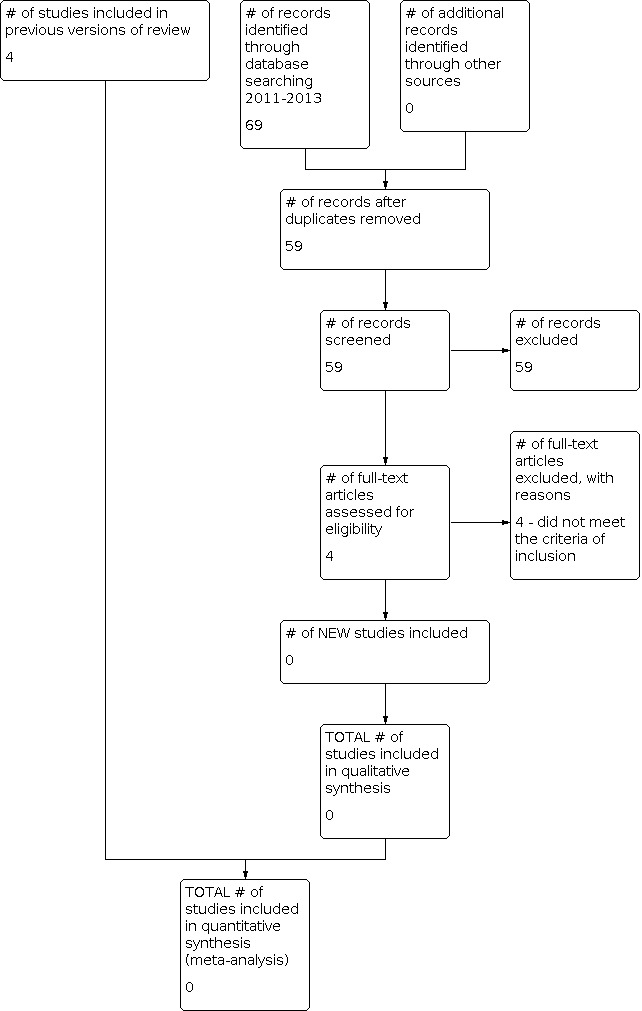
Study flow diagram.
For the original review, we examined titles and abstracts from the references identified, and found that only 27 studies related to botulinum toxin and MPS and were potentially relevant. We obtained full‐text copies of these 27 remaining studies and after assessment we found 20 RCTs, out of which five were secondary studies to two of the main studies. When checking the quality of the RCTs it was necessary to contact the authors through e‐mail to clarify doubts about the studies and also to ask them to send their data as means and standard deviations (SD), since data reported in percentages could not be included in this review. We received replies from five authors and after analysing all RCTs, we excluded 10 main studies (Benecke 2011; Cheshire 1994; Davis 2011; Esenyel 2007; Ferrante 2005; Graboski 2005; Kamanli 2005; Lew 2008; Porta 2000; Wheeler 1998) (see the Characteristics of excluded studies table). One study is still ongoing (Zeki 2010). Zeki 2010 presented preliminary results of an ongoing study, the results of which were not identified for this update; therefore, we concluded that it was still ongoing. We contacted the author and we are waiting for his confirmation (see Characteristics of ongoing studies table). Only four studies fulfilled all the inclusion criteria for this systematic review (De Andrés 2010; Göbel 2006; Ojala 2006; Qerama 2006) (see Included studies and Characteristics of included studies table).
Included studies
We included four studies (De Andrés 2010; Göbel 2006; Ojala 2006; Qerama 2006) which all researched the therapeutic effect of botulinum toxin A (BTXA). There were no included studies of botulinum toxin B.
Trial design
Two of the included studies were prospective, randomised, double‐blind, placebo‐controlled clinical trials, one (Göbel 2006) with 12 weeks of follow‐up and another (Qerama 2006) with four weeks of follow‐up. Another study (Ojala 2006) was a prospective, randomised, double‐blind and controlled study with a cross‐over strategy, where the participants received either botulinum toxin A or physiological saline injections on two occasions, four weeks apart, with the follow‐up measurements carried out at four weeks after each treatment. The last assessed study (De Andrés 2010) was a randomised, double‐blind, prospective, controlled trial with a placebo control (saline) and an active control (bupivacaine 0.25%) in participants experiencing mechanical low back pain due to bilateral MPS involving the iliopsoas or the quadratus lumborum muscles, or both. Follow‐up was at 15, 30 and 90 days.
Participants
The search strategy identified four studies with a total of 233 participants with MPS that were suitable for inclusion in the original systematic review. The diagnostic criteria used by all studies were defined by Travell 1999 and Simons 1996, except for Ojala 2006 who used Tunks 1999.
Göbel 2006
In Göbel 2006, 145 participants (80% female and 20% male; mean age 45 years) with moderate to severe MPS affecting the cervical or shoulder muscles, or both (≥ 10 trigger points, disease duration 6 to 24 months) were randomised to BTXA or saline solution groups. There were no significant differences between treatment groups in terms of demographic, physical, cardiovascular or neurological characteristics at baseline. The physician identified these trigger points by palpation of the cervical or shoulder muscles, or both. The identification was based on the operational definition of Simons 1996 and all doctors were trained for standardised trigger point identification and data collection. At the screening visit (week one), participants were given a physical examination and optional neurological and muscular tests to ensure there was no evidence of other specific disorders. Participants who had previously received treatment with botulinum toxin were not included in the study. Participants were also excluded if they presented with the following: conditions associated with medication‐induced bleeding, a body mass index of more than 30 kg/m2, or specific back pain disorders. Certain concomitant medications were not permitted during the four weeks prior to treatment (opioids, invasive therapy methods or neuromuscular blocks in the region of treatment, and parenteral or oral corticosteroids), during the week prior to treatment (NSAIDS, topical antirheumatics, topical corticosteroids, and muscle relaxants) or during the day prior to treatment (paracetamol or other analgesics, heat, massage, cold, or rheumatism bath therapy).
Qerama 2006
In Qerama 2006, the study included 30 participants (18 female and 12 male). Inclusion criteria were as follows: pain in the shoulder and referred to the arm, for more than six months duration, and associated trigger points in the infraspinatus muscle on the painful side, and minimum pain intensity of two in a numerical rating scale (NRS) of 0 to 10. Patients were randomised in a double‐blind fashion to two parallel treatment (BTXA) and control groups. Participants in the BTXA group were older (mean age (range): BTXA 54.5 (31 to 72) years, and control 46.7 (23 to 76) years, Z = ‐1.95, P = 0.051) and tended to have longer‐standing pain (mean pain duration in months: BTXA 26.3, control 13.9, Z = ‐1.801, P = 0.074). Criteria for trigger points identification were as follows:
a taut muscle band, palpable in the muscle;
a painful spot in the taut band;
recognition of current pain and sensory complaints by pressure on the painful spot;
pain and altered sensation in the distribution expected from a trigger point in the infraspinatus muscle;
painful restriction to full stretch range of motion measured by mouth wrap‐around test and hand to shoulder blade test; and
local twitch response evoked by snapping palpation of the painful spot.
The trigger point was considered stable if the first four criteria were met. Trigger point location, sex distribution and use of analgesics were similar in both groups. Participants were excluded if they had a severe psychiatric disorder, a history of severe drug allergy including previous reactions to BTXA or BTXB, or were reluctant to stop physical therapy and other manual treatment during the project period. Participants with trigger points in the ipsilateral trapezius, supraspinatus and teres muscles were also excluded.
The exclusion criteria for Göbel 2006 and Qerama 2006 were: previous or present neuromuscular disorder, history of current drug or alcohol abuse, current participation in other research studies, no use of appropriate birth control (women).
Ojala 2006
In Ojala 2006, the sample consisted of 31 participants (90% females; mean age 44.4 years). The participants had to have experienced pain in the neck‐shoulder area for over two months to be included in the empirical criteria suggested for diagnosis of MPS (Tunks 1999). Inclusion criteria included five major criteria for MPS as follows:
a regional pain complaint;
pain or paraesthesia in the typical distribution of the trigger point;
a taut band in the muscle;
exquisite tenderness found in that taut band; and
a restricted range of motion in the affected muscle.
From these, criteria 1 to 4 had to be met in all participants. The participants also had to have one of the following three minor criteria:
a reproduction of the participant’s clinical pain complaint by pressure on the trigger point;
a local twitch response in the taut band; and
alleviation of the trigger point by stretch.
The exclusion criteria included a history of diffuse pain, a history of cervical disk or bone disease, neurologic deficits, trauma of the neck‐shoulder region, previous cervical surgery, radiculopathy of the upper extremities, severe obesity and other comorbidity that could interfere with the results. Of those 57 participants who went through the screening examination, 32 fulfilled the inclusion criteria, but one person withdrew just before the first treatment. The level of depression was evaluated with the Beck Depression Inventory. The duration of pain varied considerably (range from 2 months to 30 years) but all except 3 had a history of over 1 year of pain. There were no other consecutive treatments during the study. Only paracetamol was allowed if additional medical treatment was needed during the study period, but some participants (n = 7) who temporarily used anti‐inflammatory drugs for headache (n = 2), common cold (n = 1), musculoskeletal pain in neck‐shoulder region (n = 3) or pain in some other region (n = 1) were not excluded from the final analysis.
De Andrés 2010
In De Andrés 2010, 28 participants (20 female and 8 male; mean age 51 years) with chronic low back pain due to bilateral MPS involving the iliopsoas or quadratus lumborum muscles, or both, were included in the study. One of the participants, randomised to receive bupivacaine as the control drug, refused to receive BTXA and was excluded from subsequent analysis. Inclusion criteria were as follows:
history of mechanical low back pain of longer than six months duration in participants aged from 20 to 70 years;
bilateral existence of trigger points with associated referred pain in the iliopsoas muscle, quadratus lumborum muscle, or both;
mechanical stimulation of the trigger point (using enough pressure with examiner’s finger to cause examiner’s own nail bed to blanch) inducing intense local and referred pain that is different from the pain expected on the basis of nerve root compression alone and often accompanied by withdrawal of the stimulated muscle; and
failure of conservative medical and physical therapy.
Participants with previous back surgery, spondylolisthesis, facet joints arthropathy, known or suspected hypersensitivity to botulinum toxin or its components, neurologic deficits in the painful area, neuromuscular junction or motor neuron diseases, diagnosis of fibromyalgia and inflammation or infection of the injection sites were excluded. The study was designed to exclude inter‐subject variability (each participant serving as their own control). Throughout the trial participants were allowed to continue receiving their regular medications, including anti‐inflammatory drugs, muscle relaxants and narcotics, taking care not to deviate from the prescribed regimens.
Interventions
In Göbel 2006,145 participants were randomised into two groups: one group (75 participants) received injections of BTXA (40 units per site); the second group (70 participants) received 0.9% sodium chloride solution. Injections were made by the physician into the 10 most painful trigger points.
In Qerama 2006, 30 participants were randomised into two groups: one group (15 participants) received injections of 50 units/0.25 mL of BTXA; the other group received 0.25 mL of sodium chloride (0.9%). The substances were injected at the trigger point in the infraspinatus muscles through one insertion and in five directions at four sites per direction.
In Ojala 2006, 31 participants were randomised into two groups: one group (15 participants) received injections of 5 units/0.05 mL of BTXA to each trigger point; and the other group received 0.05 mL of physiological saline to each trigger point initially. The participants received injections on two occasions in a four weeks apart cross‐over design. The follow‐up measurements were carried out at four weeks after both the first and the second injections. The total dose varied from 0.15 mL to 0.35 mL (containing 15 to 35 units of BTXA), depending on the number of active (treated) trigger points.
In De Andrés 2010, each of the 27 enrolled participants received a bilateral, fluoroscopically guided injection in the affected muscle(s) to randomly deliver 50 units/5 mL of BTXA (10 U/mL) on one side of the low back and 5 mL of control drug (randomly sodium chloride 0.9% (n = 14) or bupivacaine 0.25% (n = 13) on the opposite side. The study was designed to exclude inter‐subject variability (each participant serving as their own control).
Outcomes
In Göbel 2006, the participants were assessed in relation to the pain intensity score, which was defined as a mean weekly score of at least three points on an ordinal self rating pain scale, rated from one (no pain) to four (severe pain). For data entry and analysis of the self rating pain scale, a score ranging from 0 to 3 was used (0 = no pain, 1 = weak pain, 2 = moderate pain, 3 = severe pain) and side effects were assessed at baseline and after 12 weeks. The primary outcome was the proportion of participants with mild or no pain at week five. Secondary outcomes included changes in pain intensity and the number of pain‐free days per week. Tolerability and safety were also assessed. All participants received study medication and were included in the safety population. One participant in the BTXA group received medication but had no effectiveness data so was included in the safety population, but not in the intention‐to‐treat (ITT) population. A total of 24 participants had major protocol deviations and were excluded from the per protocol (PP) population.
In Qerama 2006, the participants were assessed at baseline after 28 days in relation to relief, pain intensity score (VAS), pressure pain detection threshold in trigger point, pressure pain tolerance in trigger point, pain and distance of mouth wrap‐around test (MWAtr) and hand to shoulder blade test (HTSHtr). The predefined primary outcome measures were median spontaneous and evoked trigger point pain intensity at the fourth visit and during the last week of follow‐up, as well as sites with motor endplate activity at the fourth visit. Secondary outcome measures were median spontaneous and evoked referred pain intensity, range of motion, pain relief, pressure pain detection and tolerance thresholds, and area of local and referred pain intensity at the fourth visit. The study authors studied the effect of BTXA on pain from muscle trigger points and on electromyography (EMG) activity at rest and during voluntary contraction. All analyses were performed by intention‐to treat (ITT) and each variable was quantified as a change from the baseline value and endpoint.
As the data were available in percentiles, making it inappropriate to include in our review, we sent an e‐mail to Dr Qerama and Dr Göbel requiring the data in means and they promptly sent us the converted data. Göbel also informed us that in his study the treatment of MPS was located on the shoulder, which made it possible for us to include his data in our systematic review.
In Ojala 2006, the participants were assessed at three time points: baseline, four weeks after the first injections, and four weeks after the second injections, and the substance used (i.e. saline or BTXA) was rated in relation to severity of neck‐shoulder pain (SNP) on a scale of 0 to 10 (endpoints: no pain (0) to unbearable pain (10)) about half an hour before each treatment session. The severity of SNP was the primary outcome parameter in this study; the participant's self assessment of the efficacy of each treatment was made using a verbal rating scale (1 = very good; 2 = rather good; 3 = some; 4 = rather small; 5 = none). The prevalence and type of adverse events of previous treatment were assessed by the participants. The minimum pressure that induces pain (PPT) of trigger points and the reference point were measured in the sitting position with a dolorimeter, expressed as kg/cm2.
In De Andrés 2010, the participants were assessed at baseline and after 15, 30 and 90 days in relation to pain intensity score using a standard VAS ranging from 0 (no pain) to 10 (unbearable pain). The VAS scores were in charts and it was not possible to extract the exact value for calculation. In this RCT there were also data for reduction in VAS scores without baseline data, thus making it impossible to calculate the VAS data to include in our systematic review. We tried to contact the authors to obtain the data, without success. To evaluate the effects of treatment on the participant’s daily life activities and psychological status they used five different questionnaires to be completed just before each visit or at the beginning and at the end of the study. These data were not used in this systematic review because each participant received both treatments, BTXA on one side and the control drug (randomly sodium chloride 0.9% or bupivacaine 0.25%) on the opposite side of the lower back. There was no effective control group of participants to allow evaluation of the real effect of the BTXA on the participant's daily life activities and psychological status compared with a control group of participants that did not receive BTXA.
For further details please see the Characteristics of included studies table.
Excluded studies
Seven RCTs did not meet the inclusion criteria as it was not possible to separate the neck data from other muscles (Benecke 2011; Cheshire 1994; Davis 2011; Ferrante 2005; Graboski 2005; Kamanli 2005; Lew 2008), one presented data related to the neck only (Wheeler 1998) and two were excluded due to high risk of bias (Esenyel 2007; Porta 2000). One study is ongoing (Zeki 2010).
For further details please see the Characteristics of excluded studies table.
Risk of bias in included studies
For risk of bias summary tables, see Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of allocation concealment was adequately described in all studies. In their studies, participants were assigned to treatment in blocks using a computer‐generated randomisation schedule.
Blinding
The four included studies (De Andrés 2010; Göbel 2006; Ojala 2006; Qerama 2006) were double‐blinded and all participants received intramuscular injections. All substances presented the same perceptible characteristics. Participants received injections during a single intervention, except for Ojala 2006 whose participants received two cross‐over interventions; as a result we just took the first follow‐up results into consideration in order to prevent bias (due to the fact that the BTXA effect lasts more than four weeks). Blinding was maintained in all studies.
In Göbel 2006, participants were assigned to treatment in blocks using a computer‐generated randomisation schedule. Injections were prepared independently by a person not involved in the study, who opened the sealed envelope containing the randomisation code and prepared the syringes accordingly with either active treatment or placebo. The randomisation code was kept centrally and unblinding took place after the end of the study, when all evaluations had been completed.
In Qerama 2006, assignment to the treatment group was also done randomly by means of a computer‐generated randomisation code known only by the assistant who had no contact with the participants during the study. Participants were enrolled and allocated by the investigator. After completion of the study the assistant, who had no contact with the participants during the study, returned the closed envelopes with the information about treatment sequence. For the reasons mentioned, we consider both studies to have a low risk of bias.
In Ojala 2006, participants were randomised to receive injections of either BTXA or physiological saline. The participants were randomised into two groups with block randomisation. The drugs needed were purchased from the pharmacy of Kuopio University Hospital, and no commercial party who had a direct or indirect interest in the patient matter was involved. The same physician performed the first stage and follow‐up measurements of the participants.
In De Andrés 2010, using a computerised double‐randomisation schedule, recruited participants were randomised to receive the same volume (5 mL) of the studied treatments in the bilaterally affected muscles: BTXA on one side and the control injection on the opposite side. The control injection was randomly sodium chloride 0.9% or bupivacaine 0.25% (randomisation ratio of 1:1). The randomisation list was stored by an investigator not involved in participant selection, treatment or evaluation and was revealed only on data analysis. All procedures were performed by the same experienced physician, who was blinded to the participants’ treatment allocation. Injection solutions were prepared by a nurse not involved in the participants’ care or follow‐up. Outcomes were evaluated by an investigator blinded to participant randomisation.
Incomplete outcome data
According to Qerama's e‐mail reply in relation to withdrawals, the main author did not perform the ITT analysis (Qerama 2006). Withdrawals were described as being less than 10% for both groups, not presenting substantial losses to the study. The data for the two participants from the BTXA group who withdrew were included in the analysis for the period they were still participating in the study. One of the participants withdrew after the third visit because of an intervening diagnosis of lung cancer. For this participant, data for spontaneous pain in the third week of treatment were based on a telephone interview. The other participant refused to undergo the motor endplate searching procedure at the fourth visit. Both participants were excluded from the motor endplate activity analysis.
According to Göbel's e‐mail reply in relation to withdrawals, the main author made the participant flow available; in this way it was possible to carry out the ITT analysis for dichotomous data. For continuous outcomes (pain, duration of sleep, physician global assessment, and systolic pressure), dropout rates for participants in the above mentioned variables were less than 3% (Göbel 2006).
In Ojala 2006, one participant withdrew before the first treatment, thus the sample consisted of 31 participants.
In De Andrés 2010, one of the participants, randomised to receive bupivacaine as the control drug, refused to receive BTXA and was excluded from subsequent analysis.
Selective reporting
The studies Göbel 2006 and Qerama 2006 used different pain scales and sent data by e‐mail after converting them. In Ojala 2006, just the first follow‐up was taken into consideration to avoid bias due to the fact that the BTXA effect lasts more than four weeks. The last study (De Andrés 2010) presented the results in graph form, precluding the use of the data for meta‐analysis.
Other potential sources of bias
We updated our methods for assessing risk of bias, and removed the single study analyses for the update in order to meet current methodological standards. We believe that the study by Göbel 2006 presented an unclear risk of bias due to the number of participants (N = 145) and Qerama 2006 presented a high risk of bias due to the number of participants (N = 30), however both were free of other problems that could lead them to be classified as at high risk of bias. In Ojala 2006, some participants kept using some kind of medication without specifying the amount and duration. In De Andrés 2010, not all data from questionnaires on QoL and global assessment were considered, since all participants received the intervention on one side with a contralateral control. Both Ojala 2006 (N = 31) and De Andrés 2010 (N = 28) presented a high risk of bias due to the number of participants.
Effects of interventions
We observed great heterogeneity in the descriptions of outcomes between studies, making it difficult to perform the meta‐analyses. In this update, the included studies did not meet the current methodological standards and therefore analyses from the original review were removed. We have added narrative descriptions for each of the outcomes below.
1. Botulinum toxin A versus placebo
Primary outcomes
1.1 Spontaneous pain intensity in trigger point (endpoint)
In the two studies that reported this outcome (Ojala 2006; Qerama 2006) there was a statistically non‐significant difference between comparison groups in the mean for spontaneous pain intensity in trigger points, measured at endpoint, that favoured botulinum toxin A (BXTA).
In another study (De Andrés 2010), the authors found no differences at any of the post‐intervention follow‐up visits. Treatment of the affected muscles with BTXA did not significantly reduce visual analogue scale (VAS) scores more than treatment of the opposite side with sodium chloride or bupivacaine.
1.2 Evoked pain intensity in trigger point (endpoint)
In the Qerama 2006 study there was a statistically non‐significant difference between comparison groups in the mean for evoked pain intensity in trigger points, measured at endpoint, that favoured placebo.
1.3 Spontaneous pain relief in trigger point (change from baseline)
The mean change from baseline between comparison groups in spontaneous pain relief in trigger point was statistically non‐significant and favoured BTXA in the Qerama 2006 study.
1.4 Evoked pain relief in trigger point (change from baseline)
The mean change from baseline between comparison groups in evoked pain relief in trigger point was statistically non‐significant and favoured placebo in the Qerama 2006 study.
1.5 Pressure pain detection threshold in trigger point (kPa) (endpoint)
There was no statistically significant difference between comparison groups in the mean for pressure pain detection threshold in trigger point, measured at endpoint, in two studies that reported this outcome (Ojala 2006; Qerama 2006).
1.6 Pressure pain tolerance in trigger point (kPa) (endpoint)
There was a statistically non‐significant difference between comparison groups in the mean for pressure pain tolerance in trigger point, measured at endpoint, that favoured BTXA in the Qerama 2006 study.
1.7 Duration of daily pain (in hours)
In the Göbel 2006 study there was a statistically significant difference between comparison groups in the mean for duration of daily pain (in hours) that favoured BTXA.
1.8 Pain intensity scores (0 to 3) (endpoint)
There was a statistically significant difference between comparison groups in the mean for pain intensity scores (0 to 3), measured at endpoint, that favoured BTXA in the Göbel 2006 study.
Secondary outcomes
1. Disability
This outcome was not reported in the included studies.
2. Quality of life (QoL) measured by Nottingham Health Profile (NHP) and SF‐36
This outcome was not reported in the included studies.
3.1 Distance (mm) ‐ mouth wrap‐around test (MWAtr) (endpoint)
In Qerama 2006 there was a statistically significant difference between comparison groups in the mean for distance (mm) on the MWAtr, measured at endpoint, that favoured placebo.
3.2 Distance (mm) ‐ hand to shoulder blade test (HTSHtr) (endpoint)
In Qerama 2006 there was a statistically significant difference between comparison groups in the mean for distance (mm) on the HTSHtr, measured at endpoint, that favoured placebo.
3.3 Distance (mm) ‐ hand to shoulder blade test (HTSHtr) (baseline)
There was a statistically non‐significant difference between comparison groups in the mean for distance (mm) on the HTSHtr, measured at baseline, that favoured placebo in the Qerama 2006 study.
3.4 Distance (mm) ‐ mouth wrap‐around test (MWAtr) (baseline)
There was a statistically non‐significant difference between comparison groups in the mean for distance (mm) on the MWAtr, measured at baseline, that favoured placebo in the Qerama 2006 study.
4. Adverse events
There were significantly more adverse events for BTXA than placebo in one of the studies (Göbel 2006). The most common adverse event was sore muscle, of mild or moderate severity, and one participant in each treatment arm withdrew from the study due to adverse events (Göbel 2006). In the other studies, the adverse event rates were similar in both groups (De Andrés 2010; Ojala 2006; Qerama 2006).
Discussion
Summary of main results
Despite the high prevalence of myofascial pain syndrome (MPS) and the possibility of the occurrence of myofascial pain in any muscle of the human body, we could find only four randomised controlled trials (RCTs) (De Andrés 2010; Göbel 2006; Ojala 2006; Qerama 2006) that fitted our inclusion criteria for this systematic review. Two studies (Göbel 2006; Qerama 2006) reported data that could be included in our systematic review after conversion from percentages to means and SDs, and one study (Ojala 2006) showed data that were converted from kg/cm² into kPa. The findings of two studies (Ojala 2006; Qerama 2006) could not be grouped due to the use of different doses of botulinum toxin (BTXA) and the findings of the other study (Göbel 2006) could not be grouped due to the use of different reported outcomes and scales. In Göbel 2006 (N = 145) a significant improvement rate with BTXA in pain intensity scores and duration of daily pain was demonstrated compared with placebo. In Qerama 2006 (N = 30) and Ojala 2006 (N = 31) there was no statistically significant difference between BTXA versus placebo in pain intensity. In De Andrés 2010 (N = 27) BTXA injection did not significantly reduce visual analogue scale (VAS) scores compared to treatment with sodium chloride or bupivacaine on the contralateral side. The treatments administered did not result in a significant improvement in participants' daily life activities or psychological status. A trend towards a decrease in post‐intervention VAS scores could be recognised in all treated low back sides of participants compared with baseline values; this trend was only significant for the sides treated with BTXA (reduction in VAS score of 20% at 15 days and 30 days, and 22% at 90 days after treatment, P < 0.05).
Overall completeness and applicability of evidence
One of the included studies (Göbel 2006) provided positive evidence for using BTXA to treat MPS whereas Qerama 2006, Ojala 2006 and De Andrés 2010 showed inconclusive effects of using BTXA in MPS. The lack of methodological standardisation influenced the validity and reproducibility of the conclusions, that is the use of different pain scales, dosages and variations in the grouping strategies in the included studies.
Quality of the evidence
Overall, the included studies had good methodological quality, but with small sample sizes and heterogeneity in the reporting of outcomes. We assessed the study by Göbel 2006 (N = 145) as being at unclear risk of bias (50 to 199 participants per treatment arm) and the studies by Qerama 2006 (N = 30), Ojala 2006 (N = 31) and De Andrés 2010 (N = 27) to be at high risk of bias (< 50 participants per treatment arm).
Potential biases in the review process
In Qerama 2006 the participants' evaluations were reported using a numerical rating scale (NRS) of 0 to 10, but in a reply to our e‐mail the author provided us with the data in a VAS of 0 to 100. This conversion does not represent a significant change according to Tubergen 2002 and Hollen 2005, however there is some controversy according to some authors (such as Daoust 2008). Göbel's study used a verbal rating scale (VRS) of 1 to 4 and for data entry and analysis of the self rating pain scale used a score ranging from 0 to 3, as the author informed us through e‐mail. A study performed by Cork 2004 showed an excellent correlation between the VRS (1 = no pain, 2 = mild pain, 3 = moderate pain, 4 = severe pain) and the VAS (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain), although there was a tendency for the VRS to be higher than the VAS, with a P value of 0.068. It is preferable to use a wider‐range scale since wide limits of agreement and variable bias lower the validation of the study. The uncertainty of Göbel's study in relation to what was included in the pain intensity score (information recorded in the participants's diary, palpation performed by the physician, or both), and because Qerama's findings were presented in subgroups, we were unable to perform a meta‐analysis.
In Qerama 2006 participants in the BTXA group were older and those participants in the placebo control tended to have longer‐standing pain. The ideal is for the treatment and control groups to be as homogenous as possible so that the study has a similar baseline between groups, such as in the Göbel 2006 study. In Ojala 2006, we took just the first follow‐up into consideration to avoid bias, due to the fact that the BTXA effect lasts more than four weeks. In De Andrés 2010, the VAS scores were in charts and it was not possible to determine the exact value from them for calculation; there were also data for reduction in VAS scores without baseline data. The data for effects of treatment on participants’ daily life activities and psychological status at the beginning and at the end of the study were measured using five different questionnaires, and were not used in this systematic review because there was no effective control group to allow evaluation of the real effect of BTXA compared with a group of participants that did not receive BTXA.
Agreements and disagreements with other studies or reviews
A systematic review evaluating treatment of neck myofascial pain with BTXA is already available (Peloso 2007). Peloso 2007 found six high‐quality studies, among which four studies with a total of 139 participants were included in a meta‐analysis. It was found that there is moderate evidence that BTXA injections are not superior to saline for chronic neck disorders with or without radicular findings or headache.
In Göbel 2006, the findings for pain intensity were more favourable towards the use of BTXA in contrast to the conclusions of Qerama 2006, Ojala 2006, De Andrés 2010 and Peloso 2007. In Göbel 2006, the sample size was 145 participants and ITT analysis was realised in all randomised participants. Dropout rates for the above mentioned variables were less than 3%. It would be interesting if a study with a larger number of participants and ITT analysis could be realised in order to increase statistical power. Despite the fact that the sample size in Göbel 2006 was greater, the pain scale used a range of 0 to 3, which led to a reduced spectrum of answers. On the other hand, Qerama 2006, Ojala 2006 and De Andrés 2010 used a valid pain scale showing results with better statistical power. However, the studies involved few participants.
In De Andrés 2010, each of the 27 participants received a bilateral, fluoroscopically guided injection in the affected muscles with BTXA on one side of the low back and a control drug (randomly sodium chloride 0.9% or bupivacaine 0.25%) on the opposite side. Although there was a trend toward a decrease in post‐intervention VAS scores reported by the participants in the sides treated with BTXA, it was observed in this study that BTXA injection did not significantly reduce VAS scores more than treatment with sodium chloride or bupivacaine in the contralateral side. Unfortunately, the data from this RCT could not be included in this review due to the lack of baseline data and the presentation of the data in charts.
In Graboski 2005, a double‐blind, randomised, cross‐over trial with 18 participants, there was no significant difference between the BTXA and 0.5% bupivacaine groups in duration or magnitude of pain relief, function or participant satisfaction. In Lew 2008 (N = 29), the author observed a trend toward improvement in the VAS and Neck Disability Index (NDI) scores in the BTXA group but it was not significant. In Ojala 2006 (N = 31), the authors concluded that there was no difference between the effect of small doses of BTXA and that of physiological saline in the treatment of MPS.
The maximum effect of botulinum toxin injection occurs from two to six weeks and it reduces gradually. Effectiveness can vary from two to six months (Bigalke 2003; Reilich 2003). In Qerama 2006, follow‐up was only 28 days after injection. In Ojala 2006, the first follow‐up was at four weeks, providing the data used in this review. In Göbel 2006, the follow‐up period was up to 12 weeks. It would be better for future studies to extend the period of follow‐up to six months to assess the maximum effect of the medicine as well as its decrease over time.
Botulinum toxin can be used to reduce muscular spasm, aiming to facilitate rehabilitation measures, as well as leading to prolonged muscle relaxation when compared to other substances used in MPS such as trigger point infiltration with topical analgesic (Lin 2003). In Hubbard 1993, the authors hypothesise that trigger points are caused by sympathetically activated intrafusal contractions. In Simons 2002 endplate noise was significantly more prevalent in myofascial trigger points than in sites that were outside of a trigger point but still within the endplate zone. Chou 2009 suggested a correlation between endplate activity and pain at the trigger point, but that does not seem to be the only correlation since Qerama 2006 showed no significant reduction of pain with the lowering of the motor endplate activity. In Qerama 2006, BTXA administered in the trigger point of the muscle showed a trend toward improving the range of motion of the muscle and reducing the area of pain, with no significant differences. These results indicate that although the toxin altered motor activity, it did not significantly influence the sensory processing in the muscle.
The dose of BTXA used in the four included studies might be considered too low to have a perceptible effect on pain (from 5 to 50 units). The dose used in earlier studies of pain conditions varied between 25 and 100 units/site with the highest dose being associated with more frequent side effects (Silberstein 2000). Wheeler 1998 evaluated two dosage levels (50 units and 100 units) and found these to be equivalent to each other and to placebo. In Göbel 2006, the dose of BTXA used was 40 Ipsen units per site. In Ojala 2006 the dose of BTXA used was very small (5 units/site). In Qerama 2006 and De Andrés 2010 the BTXA dose used was 50 units/site. The ideal dose of botulinum toxin for treating different types and sizes of muscles should be standardised.
The needling procedure has been shown to have an effect on muscle trigger points (Kamanli 2005; Travell 1999) and all included studies used the standardised needle procedure in both groups.
Further high‐quality RCTs of botulinum toxin for treating MPS need to be conducted before firm conclusions on its effectiveness and safety can be drawn.
We believe that in the future, when more studies are added to our review, we will be able to make more substantial judgements on the evidence available.
Authors' conclusions
Implications for practice.
No new relevant studies were found since the last version of this review (see Figure 1). The limited available evidence, involving a total of 233 participants from four studies, suggests that there was some improvement in pain intensity, duration of daily pain and more side effects in the botulinum toxin A (BTXA) group. There is currently only limited evidence to support the use of botulinum toxin in the treatment of myofascial pain syndrome (MPS).
Implications for research.
There is a need for more randomised controlled trials that have a bigger number of participants (≥ 200 participants per treatment arm) and are at low risk of bias. It is also necessary to use validated scales in order to increase the external validity of the findings (applicability). Moreover, the diversity of ways of reporting estimate effects should be standardised by using clinically relevant and objective outcome variables, including quality of life measures. These are needed to establish the true therapeutic effects of BTXA and, if that is shown, further studies would be required to establish the best dose and type of drug to use. Further studies that include rigorous, multi‐domain follow‐up of participants receiving treatment are needed to determine the long‐term effects of BTXA.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2021 | Review declared as stable | No longer being updated. See Published notes. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 28 July 2014 | Review declared as stable | This review will be assessed for further updating in 2019. |
| 5 April 2014 | New citation required but conclusions have not changed | No new studies were identified for inclusion in this update, and therefore the conclusions remain unchanged. |
| 5 April 2014 | New search has been performed | Searches updated in December 2013, and risk of bias summary tables added. |
| 27 June 2012 | Amended | Contact details updated. |
Notes
This review will no longer be updated. It is correct at the time of publication and is for historical interest only.
Acknowledgements
For the original review, we would like to thank the Brazilian Cochrane Centre staff; the precious support of Jessica Thomas, Cochrane Pain, Palliative and Supportive Care (PaPaS) Group Managing Editor; Caroline Struthers, Trials Search Co‐ordinator, as well as Jane Hayes, of the PaPaS Review Group, for the search strategy for identifying RCTs; and, finally, the physiotherapist Ane Helena Valle Versiani for her input in helping conceive the clinical question presented in this systematic review. We also thank the authors who have promptly and gently responded to our e‐mails, especially Drª Erisela Qerama and Hartmut Göbel.
Appendices
Appendix 1. Search strategies used for 2013 update
CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Myofascial Pain Syndromes] explode all trees
#2 MeSH descriptor: [Temporomandibular Joint Disorders] explode all trees
#3 ("myofascial pain syndrome" or synalg* or (myofascia* near/6 pain* near/6 syndrome*)):it,ab,kw (Word variations have been searched)
#4 (myofascia* and ("trigger point*" or trigger‐point*)):it,ab,kw (Word variations have been searched)
#5 (pain* near/6 (myofascia* or focal or focus* or referr* or local*)):it,ab,kw (Word variations have been searched)
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Botulinum Toxins] explode all trees
#8 (Botulinum and (neurotoxin* or toxin*)):it,ab,kw (Word variations have been searched)
#9 (Botulinum and (A or B or C or C1 or C2 or C‐1 or C‐2 or D or E or F or G)):it,ab,kw (Word variations have been searched)
#10 (Botox or BTX or dysport or neurobloc or oculinum or vistabel or myobloc):it,ab,kw (Word variations have been searched)
#11 #7 or #8 or #9 or #10
#12 #6 and #11
1. ("botulinum toxin" or botulinum‐toxin) and pain
2. myofasc*
3. 1 and 2
MEDLINE (Ovid)
1. exp Myofascial Pain Syndrome/ or exp Temporomandibular Joint Disorders/ 2. ("myofascial pain syndrome" or synalg* or (myofascia* adj6 pain* adj6 syndrome*)).mp. 3. (myofascia* and ("trigger point*" or trigger‐point*)).mp. 4. (pain* adj6 (myofascia* or focal or focus* or referr* or local*)).mp. 5. or/1‐4 6. botulinum toxin/ or botulinum toxin a/ or botulinum toxin b/ or botulinum toxin e/ or botulinum toxin f/ 7. Clostridium Botulinum Type B/ or Clostridium Botulinum Type D/ or Clostridium Botulinum/ or Clostridium Botulinum Type C/ or Clostridium Botulinum Type E/ or Clostridium Botulinum Type F/ or Clostridium Botulinum Type A/ 8. (Botox or BTX or dysport or neurobloc or oculinum or vistabel or myobloc).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 9. (Botulinum and (neurotoxin* or toxin* or A or B or C or C1 or C2 or C‐1 or C‐2 or D or E or F or G)).mp. 10. or/6‐9 11. 5 and 10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. drug therapy.fs. 17. randomly.ab. 18. trial.ab. 19. or/12‐18 20. exp animals/ not humans.sh. 21. 19 not 20 22. 11 and 21 23. (2012* or 2013*).ed. 24. 22 and 23
EMBASE (Ovid)
1. exp Myofascial Pain Syndrome/ or exp Temporomandibular Joint Disorders/
2. ("myofascial pain syndrome" or synalg* or (myofascia* adj6 pain* adj6 syndrome*)).mp.
3. (myofascia* and ("trigger point*" or trigger‐point*)).mp.
4. (pain* adj6 (myofascia* or focal or focus* or referr* or local*)).mp.
5. or/1‐4
6. botulinum toxin/ or botulinum toxin a/ or botulinum toxin b/ or botulinum toxin e/ or botulinum toxin f/
7. Clostridium Botulinum Type B/ or Clostridium Botulinum Type D/ or Clostridium Botulinum/ or Clostridium Botulinum Type C/ or Clostridium Botulinum Type E/ or Clostridium Botulinum Type F/ or Clostridium Botulinum Type A/
8. (Botox or BTX or dysport or neurobloc or oculinum or vistabel or myobloc).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
9. (Botulinum and (neurotoxin* or toxin* or A or B or C or C1 or C2 or C‐1 or C‐2 or D or E or F or G)).mp.
10. or/6‐9
11. 5 and 10
12. random$.tw.
13. factorial$.tw.
14. crossover$.tw.
15. cross over$.tw.
16. cross‐over$.tw.
17. placebo$.tw.
18. (doubl$ adj blind$).tw.
19. (singl$ adj blind$).tw.
20. assign$.tw.
21. allocat$.tw.
22. volunteer$.tw.
23. Crossover Procedure/
24. double‐blind procedure.tw.
25. Randomized Controlled Trial/
26. Single Blind Procedure/
27. or/12‐26
28. (animal/ or nonhuman/) not human/
29. 27 not 28
30. 11 and 29
31. (2012* or 2013*).dd.
32. 30 and 31
LILACS
botulín$ or botulin$ [Palavras] and myofasc$ or miofasc$ or pain [Palavras]
No study design filter was applied.
Appendix 2. Table of searches
| Version | Date of search | Records retrieved | RCT/CCT filter applied | Records retrieved | Duplicates | Records sent | |
| 1. PaPaS Specialised Register | 18 May 2009 | 18 May 2009 | 21 | RCT/CCT | 21 | 1 | 20 |
| 2. The Cochrane Library | 2009, Issue 2 | 18 May 2009 | 33 | CENTRAL | 27 | 21 | 6 |
| COCHRANE REVIEWS | 2 | 2 | |||||
| DARE (other reviews) | 3 | 3 | |||||
| 3. MEDLINE (Ovid) | 1950 to May week 2 2009 | 18 May 2009 | 164 | Cochrane filter | 140 | 23 | 117 |
| In‐Process & Other Non‐Indexed Citations 15 May 2009 | 18 May 2009 | 5 | No | 5 | 0 | 5 | |
| 4. EMBASE (Ovid) | 1980 to 2009 week 20 | 18 May 2009 | 338 | Yes | 63 | 33 | 30 |
| 5. LILACS | 1982 to present | 18 May 2009 | 13 | no | 13 | 1 | 12 |
| Totals | 274 | 79 | 195 |
Appendix 3. Table of updated searches ‐ December 2011
| Version | Date of search | Records sent | |
| 1. The Cochrane Library | 2011, Issue 4 | 15 December 2011 | 9 |
| 2. MEDLINE (Ovid) | May Week 2 2009 to November week 3 2011 | 15 December 2011 | 43 |
| 3. EMBASE (Ovid) | 2009 Week 20 to 2011 week 49 | 15 December 2011 | 144 |
| 4. LILACS | May 2009 to December 2011 | 15 December 2011 | 10 |
| Totals | 206 |
Appendix 4. Table of updated searches ‐ December 2013
| Database searched | Date searched | Number of results |
| CENTRAL (The Cochrane Library) Issue 11 of 12, 2013 (de‐duplicated against 2010 results) |
3/12/13 | 13 |
| MEDLINE (OVID) 2012 to 29/11/13 | 03/12/13 | 34 |
| EMBASE (OVID) 2012 to 27/11/13 | 03/12/13 | 17 |
| LILACS (Bireme) 2012 to May 2014 | 13/5/14 | 5 |
| Total | 69 | |
| After de‐duplication | 59 | |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Andrés 2010.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, controlled trial Intention‐to‐treat: not used Sample size calculation: "to detect a clinically significant reduction of at least 2 VAS points (25%) in pain scores of side treated with BTXA versus those treated with placebo. A sample of 26 participants would be sufficient, with 0.8 probability (power) and an > error of 0.05. To account for possible dropouts, we planned to enroll 28 patients in this trial". Setting: Multidisciplinary Pain Management Center of Valencia University General Hospital Follow‐up: 90 days |
|
| Participants | N = 28 (8 male, 20 female) Mean age (years): 51 ± 12 Mean weight (kg): 66 ± 12 One patient (randomised to receive bupivacaine as the control drug) later refused to receive BTXA and was excluded from subsequent analysis |
|
| Interventions | Group 1 ‐ BTXA (10 U/mL), n = 27 Group 2 ‐ placebo: 0.9% NaCl solution, n = 14 Group 3 ‐ placebo: bupivacaine 0.25%, n = 13 Each participant received a bilateral, fluoroscopically guided injection in the affected muscle(s) to randomly deliver BTXA on one side of the low back and a control injection on the contralateral side, randomly NaCl or bupivacaine |
|
| Outcomes | 1. Pain intensity score (VAS) 2. Daily life activities and psychological status 3. Adverse events |
|
| Notes | Iliopsoas and/or the quadratus lumborum muscles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a computerized double‐randomisation schedule, recruited patients were randomised...(randomisation ratio of 1:1)." |
| Allocation concealment (selection bias) | Low risk | "The randomisation list was stored by an investigator uninvolved in patient selection, treatment, or evaluation and was revealed only on data analysis." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All procedures were performed by the same experienced physician, who was blinded to the patients’ treatment allocation, under continuous fluoroscopic imaging guidance. Injection solutions were prepared by a nurse uninvolved in the patients’ care or follow‐up; BTX‐A is colourless and visually identical to bupivacaine 0.25% or NaCl 0.9%." "Outcomes were evaluated by an investigator blinded to patient randomisation." |
| Selective reporting (reporting bias) | High risk | The De Andrés data were not used in this systematic review because the VAS scores are in charts and it is not possible to remove from them the exact value for calculation. In this RCT there are also data for reduction in VAS scores without baseline data, thus making it impossible to calculate the VAS data to include in our systematic review. |
| Other bias | High risk | The data for treatment effects on the participants’ daily life activities and psychological status at the beginning and at the end of the study were measured by 5 different questionnaires and were not used in this systematic review. The same participant received both treatments, randomly delivered BTXA in one side and the control drug (randomly NaCl 0.9% or bupivacaine 0.25%) in the opposite side of the lower back and then the participant was asked to answer the questionnaires mentioned above. There was no effective control group to allow evaluation of the real effect of BTXA on the participants' daily life activities and psychological status compared to a control group of participants that did not receive BTXA. |
| Size of study | High risk | N = 28 participants |
Göbel 2006.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, placebo‐controlled, multicentre study Intention‐to‐treat: used Sample size calculation: "calculated assuming a difference of 30% between active treatment and placebo (performed with N, idv, Gauting, Germany)" Setting: multicentre (15 hospitals from Germany and Austria) Follow‐up: 12 weeks |
|
| Participants | N = 145 (20% male, 80% female) Mean age (years): 44.5 Mean weight (kg): 71 |
|
| Interventions | Group 1 ‐ botulinum type A toxin: injections were made by the physician into the 10 most painful trigger points (40 Ipsen units per site), n = 75 Group 2 ‐ placebo: 0.9% NaCl solution, n = 70 |
|
| Outcomes | 1. Pain intensity score 2. Duration of daily pain (in hours) 3. Duration of sleep (in hours) 4. Number of trigger points over the course of the study 5. Mean pain intensity scores for all trigger points 6. Physicians’ global assessment of the patient’s condition 7. Adverse events: sore muscle |
|
| Notes | Upper back myofascial pain syndrome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were assigned to treatment in blocks using a computer‐generated randomizations schedule." |
| Allocation concealment (selection bias) | Low risk | "Injections were prepared independently by a person not involved in the study, who opened the sealed envelope containing the randomizations code and prepared the syringes accordingly with either active treatment or placebo. The randomizations code was kept centrally, and unbinding took place after the end of the study, when all evaluations had been completed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding was maintained until the data analysis was completed |
| Selective reporting (reporting bias) | Low risk | The reports of the study were free of suggestion of selective outcome reporting |
| Other bias | Low risk | It appears that the study was free of other problems that could put it at a high risk of bias |
| Size of study | Unclear risk | N = 145 participants |
Ojala 2006.
| Study characteristics | ||
| Methods |
Study design: double‐blind, prospective, randomised and controlled cross‐over trial Intention‐to‐treat: not used Sample size calculation: not used Setting: Finland Follow‐up: 4 weeks after each treatment |
|
| Participants | N = 31(10% male, 90% female) Mean age (years): 44.4 ± 7.7 Mean duration of neck pain (years): 10 ± 8.6 Mean BMI (kg/m2): 23.4 ± 2.6 |
|
| Interventions | Group 1 ‐ botulinum toxin A (BTXA) 5 U/trigger point Group 2 ‐ placebo ‐ NaCl solution The volume of each injection was 0.05 mL of either normal saline without preservative or saline containing 5 units of BTXA. The total dose varied from 15 to 35 U of BTXA, total volume 0.15 to 0.35 mL |
|
| Outcomes | 1. Subjective severity of neck–shoulder pain (SSNP) ‐ using questionnaires 2. Pressure‐pain threshold (PPT) ‐ using the dolorimeter 3. Subjective assessment of the efficacy of the treatment (assessed with questionnaires) |
|
| Notes | The treated muscles included the trapezius, levator scapulae and infraspinatus, bilaterally | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomised into two groups by a block randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, but not specified whether it is so throughout the process |
| Selective reporting (reporting bias) | Low risk | The cross‐over results were added up, but we considered just the first follow‐up to prevent bias |
| Other bias | High risk | Use of medication by some participants during the study |
| Size of study | High risk | N = 31 participants |
Qerama 2006.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, parallel study Intention‐to‐treat: not used Sample size: "Pain reduction of 25 to 30% from baseline has been considered clinically relevant. Expecting the mean pain intensity at the baseline correspond to a 25 to 30% reduction of the baseline.Thus, a total of 26 participants were expected to be sufficient to obtain a statistical power greater than 90% (α = 0.05) for a parallel trial." Setting: Department of Neurophysiology, Aarhus University Hospital, Denmark Follow‐up: 28 days |
|
| Participants | N = 30 (40% male, 60% female) Mean age (years): BTXA 54.5, control 46.7 Mean pain duration (months): BTXA 26.3, control 13.9 Trigger point location, sex distribution and use of analgesics were similar in both groups |
|
| Interventions | Group 1 ‐ botulinum toxin A (BTXA) ‐ 50 units/0.25 mL of BTXA Group 2 ‐ placebo ‐ 0.25 mL of sodium chloride (0.9%) For both comparison groups units were administered via the EMG needle at the second visit. The substances were injected at the trigger point through one insertion and in 5 directions at 4 sites per direction |
|
| Outcomes | 1. Median spontaneous and evoked trigger point pain intensity 2. Motor endplate activity 3. Range of motion 4. Pain relief 5. Pressure pain detection and tolerance thresholds 6. Median spontaneous and evoked referred pain intensity 7. Area of local and referred pain intensity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignment to treatment group was done randomly by means of a computer generated randomizations code, known only by the assistant (LF), who had no contact with the participants during the study." |
| Allocation concealment (selection bias) | Low risk | Participants were enrolled and allocated by the investigator (EQ). After completion of the study the assistant (LF) who had no contact with the participants during the study, returned the closed envelopes with information about treatment sequence. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding was maintained until the data analysis was completed |
| Selective reporting (reporting bias) | Low risk | The reports of the study were free of suggestion of selective outcome reporting |
| Other bias | Low risk | It appears that the study was free of other problems that could put it at a high risk of bias |
| Size of study | High risk | N = 30 participants |
BMI: body mass index BTXA: botulinum toxin A RCT: randomised controlled trial U: units VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benecke 2011 | RCT included head and neck. The pain outcomes had all the muscle groups included (upper back, neck and head); thus, it was not possible to separate them to include in this review |
| Cheshire 1994 | RCT ‐ cervical paraspinal and shoulder girdle muscles |
| Davis 2011 | RCT ‐ the most commonly injected muscles were the bilateral splenius capitis (neck muscles) |
| Esenyel 2007 | Quasi‐randomised. No blinding among the groups. High risk of bias |
| Ferrante 2005 | RCT ‐ neck and shoulder muscles |
| Graboski 2005 | RCT ‐ myofascial pain syndrome with trigger points in the neck, shoulder girdle, hip girdle or back regions |
| Kamanli 2005 | RCT ‐ randomisation was performed without specifying the method used, single‐blind. Cervical and/or periscapular regions. We sent Kamanli an e‐mail in September 2009 to clarify the randomisation process, the allocation concealment, as well as whether there was any loss to follow‐up and if the withdrawals were included in an intention‐to‐treat analysis, but we did not receive a reply |
| Lew 2008 | RCT ‐ neck and upper back pain |
| Porta 2000 | RCT ‐ participants received either BTXA in saline (n = 20) or depot methylprednisolone (n = 20), both with 0.5% bupivacaine. The intensive post injection physiotherapy programme was an important part of the treatment protocol, however 7 out of 20 participants from the steroid group did not complete the physiotherapy programme which introduced high risk of bias. Porta 2000 was considered the main study because it has more complete information. Three secondary studies from 1999, one about tension‐type headache and 2 others about myofascial pain syndrome were also excluded for the same reason as the main study |
| Wheeler 1998 | RCT ‐ myofascial pain in the neck region |
BTXA: botulinum toxin A RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Zeki 2010.
| Study name | The effectiveness of botulinum toxin type‐A and prilocaine injections in myofascial pain syndrome [abstract] |
| Methods |
Study design: prospective, randomised controlled trial Intention‐to‐treat: not shown Sample size calculation: not shown Setting: not shown Follow‐up: 6 weeks The assessments were performed at baseline and at the 2nd and 6th weeks after the injections |
| Participants | N = 46 BTXA group (n = 23) and prilocaine group (n = 23) The study finished with 38 patients (19 patients in each group) |
| Interventions | Group 1 ‐ botulinum toxin A (BTXA) ‐ 10 U of BTXA Group 2 ‐ active placebo ‐ 2 mL prilocaine The injections were made to the most painful (maximum 3) trigger points of the patients |
| Outcomes | Clinical assessment parameters were
1. Visual analogue scale (VAS) for pain during function 2. Pain score (PS) during digital pressure 3. Short form‐36 (SF‐36) for quality of life 4. Beck Depression Inventory (BDI) for general well‐being |
| Starting date | Not shown |
| Contact information | Not shown |
| Notes | All of the patients were trained in the stretching exercises and instructed to do them at home regularly |
BTXA: botulinum toxin A U: units VAS: visual analogue scale
Differences between protocol and review
None.
Contributions of authors
Adriana Soares: conception of review, searching for studies, selection of studies, data extraction, writing and updating future versions of this review.
Edina Mariko Koga da Silva: methodological support for the protocol, selection of studies, data extraction and statistical advice.
Regis B Andriolo: inputting data into RevMan 5 (RevMan 2012).
Álvaro N Atallah: methodological support of the review.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
De Andrés 2010 {published data only}
- De Andrés J, Adsuara VM, Palmisani S, Villanueva V, López-Alarcón MD. A double-blind, controlled, randomised trial to evaluate the efficacy of botulinum toxin for the treatment of lumbar myofascial pain in humans. Regional Anesthesia and Pain Medicine 2010;35(3):255-60. [DOI] [PubMed] [Google Scholar]
Göbel 2006 {published and unpublished data}
- Göbel H, Heinze A, Reichel G, Hefter H, Benecke R. Dysport myofascial pain study group. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomised double-blind placebo-controlled multicentre study. Pain 2006;125(1-2):82-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ojala 2006 {published data only}
- Ojala T, Arokoski JP, Partanen J. The effect of small doses of botulinum toxin A on neck-shoulder myofascial pain syndrome: a double-blind, randomised, and controlled crossover trial. Clinical Journal of Pain 2006;22(1):90-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ojala TA, Arokoski JP, Partanen JV. Needle-electromyography findings of trigger points in neck-shoulder area before and after injection treatment. Journal of Musculoskeletal Pain 2006;14(1):5-14. [Google Scholar]
- Ojala TA, Jurvelin JS, Partanen JV, Arokoski JP. Soft tissue stiffness before and after trigger point injections in neck-shoulder myofascial pain syndrome: a double-blind, randomized, and controlled crossover trial with botulinum toxin A and saline injections. Journal of Musculoskeletal Pain 2010;18(1):38-48. [DOI: 10.3109/10582450903496021] [EMBASE: 2010166918] [DOI] [Google Scholar]
Qerama 2006 {published and unpublished data}
- Qerama E, Fuglsang-Frederiksen A, Kasch H, Bach FW, Jensen TS. A double-blind, controlled study of botulinum toxin A in chronic myofascial pain. Neurology 2006;67:241-5. [EMBASE: 44305406] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benecke 2011 {published data only}
- Benecke R, Heinze A, Reichel G, Hefter H, Gobel H. Botulinum type A toxin complex for the relief of upper back myofascial pain syndrome: how do fixed-location injections compare with trigger point-focused injections? Pain Medicine 2011;12(11):1607-14. [DOI] [PubMed] [Google Scholar]
Cheshire 1994 {published data only}
- Cheshire WP, Abashian SW, Mann JD. Botulinum toxin in the treatment of myofascial pain syndrome. Pain 1994;59:65-9. [DOI] [PubMed] [Google Scholar]
Davis 2011 {published data only}
- Davis TL, Fang JY, Gill CE, Charles PD. A pilot trial of onabotulinumtoxinA for the treatment of cervicothoracic myofascial pain. Botulinum Journal 2011;2(1):6-15. [Google Scholar]
Esenyel 2007 {published data only}
- Esenyel M, Aldemir T, Gursoy E, Esenyel CZ, Demir S, Durmusoglu G. Myofascial pain syndrome: efficacy of different therapies. Journal of Back and Musculoskeletal Rehabilitation 2007;20(1):43-7. [Google Scholar]
Ferrante 2005 {published data only}
- Ferrante FM, Bearn L, Rothrock R, King L. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology 2005;103(2):377-83. [DOI] [PubMed] [Google Scholar]
Graboski 2005 {published data only}
- Graboski CL, Shaun Gray D, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomised double blind crossover study. Pain 2005;118(1-2):170-5. [DOI] [PubMed] [Google Scholar]
Kamanli 2005 {published data only}
- Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatology International 2005;25(8):604-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lew 2008 {published data only}
- Lew HL, Lee EH, Castaneda A, Klima R, Date E. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Archives of Physical Medicine and Rehabilitation 2008;89(1):75-80. [DOI] [PubMed] [Google Scholar]
Porta 2000 {published data only}
- Porta M, Loiero M, Gamba M, Luccarelli G, Fornari M. The use of botuline A toxin in the treatment of myofascial painful syndromes. Riabilitazione 1999;32(2):49-55. [Google Scholar]
- Porta M, Loiero M, Gamba M. Botulinum toxin type a for myofascial pain syndromes: a comparative trial versus methylprednisolone. European Journal of Neurology 1999;6 Suppl 3:107. [Google Scholar]
- Porta M. A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of myofascial pain syndrome and pain from chronic muscle spasm. Pain 2000;85(1-2):101-5. [EMBASE: 3011074] [DOI] [PubMed] [Google Scholar]
- Porta M. Botulinum toxin type A injections for myofascial pain syndrome and tension-type headache. European Journal of Neurology 1999;6 Suppl 4:S103-9. [Google Scholar]
Wheeler 1998 {published data only}
- Wheeler AH, Goolkasian P, Gretz SS. A randomised, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine 1998;23(15):1662-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Zeki 2010 {published data only}
- Zeki K, Mehmet O, Baki C, Engin D, Oguz T, Levent D, et al. The effectiveness of botulinum toxin type - A and prilocaine injections in myofascial pain syndrome. Arthritis and Rheumatism 2010;62 Suppl 10:114. [DOI: 10.1002/art.27883] [DOI] [Google Scholar]
Additional references
Argoff 2002
- Argoff CE. A focused review on the use of botulinum toxins for neuropathic pain. Clinical Journal of Pain 2002;18 Suppl 6:S177-81. [PMID: 12569966] [DOI] [PubMed] [Google Scholar]
Baldry 2002
- Baldry P. Management of myofascial trigger point pain. Acupuncture in Medicine: Journal of the British Medical Acupuncture Society 2002;20(1):2-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bigalke 2003
- Bigalke H, Naumann M. Pharmacology of botulinum neurotoxins. In: Jost WH, editors(s). Botulinum Toxin in Painful Diseases. Pain Headache. Vol. 14. Basel: Karger, 2003:1-13. [Google Scholar]
Borg‐Stein 2006
- Borg-Stein J. Treatment of fibromyalgia, myofascial pain, and related disorders. Physical Medicine and Rehabilitation Clinics of North America 2006;17(2):491-510. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borodic 2001
- Borodic GE, Acquadro M, Johnson EA. Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opinion on Investigational Drugs 2001;10:1531-44. [DOI] [PubMed] [Google Scholar]
Brashear 1999
- Brashear A, Lew MF, Dykstra DD, Comella CL, Factor SA, Rodnitzky RL. Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A–responsive cervical dystonia. Neurology 1999;53:1439-46. [DOI] [PubMed] [Google Scholar]
Bron 2007
- Bron C, Wensing M, Franssen JL, Oostendorp RA. Treatment of myofascial trigger points in common shoulder disorders by physical therapy: a randomised controlled trial [ISRCTN75722066]. BMC Musculoskeletal Disorders 2007;5(8):107. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 2009
- Chou LW, Hsieh YL, Kao MJ, Hong CZ. Remote influences of acupuncture on the pain intensity and the amplitude changes of endplate noise in the myofascial trigger point of the upper trapezius muscle. Archives of Physical Medicine and Rehabilitation 2009;90(6):905-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cork 2004
- Cork RC, Isaac I, Elsharydah A, Saleemi S, Zavisca F, Alexander L. A comparison of the verbal rating scale and the visual analog scale for pain assessment. The Internet Journal of Anesthesiology 2004;8(1):1. [Google Scholar]
Daoust 2008
- Daoust R, Beaulieu P, Manzini C, Chauny JM, Lavigne G. Estimation of pain intensity in emergency medicine: a validation study. Pain 2008;138(3):565-70. [DOI] [PubMed] [Google Scholar]
Dundar 2007
- Dundar U, Evcik D, Samli F, Pusak H, Kavuncu V. The effect of gallium arsenide aluminium laser therapy in the management of cervical myofascial pain syndrome: a double blind, placebo-controlled study. Clinical Rheumatology 2007;26(6):930-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwards 2005
- Edwards J. The importance of postural habits in perpetuating myofascial trigger point pain. Acupuncture in Medicine: Journal of the British Medical Acupuncture Society 2005;23(2):77-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ernberg 2002
- Ernberg M. The physiopathological mechanisms behind chronic myofacial pain. Lakartidningen 2002;99(32-3):3206-10. [PMID: ] [PubMed] [Google Scholar]
Esenyel 2000
- Esenyel M, Caglar N, Aldemir T. Treatment of myofascial pain. American Journal of Physical Medicine and Rehabilitation 2000;79(1):48-52. [DOI] [PubMed] [Google Scholar]
Fishbain 1986
- Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patient's categories by DSM-III psychiatric diagnostic criteria. Pain 1986;26:181-97. [DOI] [PubMed] [Google Scholar]
Flor 1993
- Flor H, Birbaumer N. Comparison of the efficacy of electromyographic biofeedback, cognitive-behavioral therapy, and conservative medical interventions in the treatment of chronic musculoskeletal pain. Journal of Consulting and Clinical Psychology 1993;61(4):653-8. [DOI] [PubMed] [Google Scholar]
Fricton 1985
- Fricton JR, Kroening R, Haley D, Siegert R. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surgery 1985;60:615-23. [DOI] [PubMed] [Google Scholar]
Fricton 1994
- Fricton J. Myofascial pain. Baillière's Clinical Rheumatology 1994;8(4):857-80. [DOI] [PubMed] [Google Scholar]
Gerwin 1995
- Gerwin RD. A study of 96 subjects examined both for fibromyalgia and myofascial pain [Abstract]. Journal of Musculoskeletal Pain 1995;3 Suppl 1:121. [Google Scholar]
Guststein 1938
- Guststein M. Diagnosis and treatment of muscular rheumatism. British Journal of Physical Medicine 1938;1:302-21. [Google Scholar]
Han 1997
- Han SC, Harrison P. Myofascial pain syndrome and trigger-point management. Regional Anesthesia 1997;22(1):89-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hanten 2000
- Hanten WP, Olson SL, Butts NL, Nowicki AL. Effectiveness of a home program of ischemic pressure followed by sustained stretch for treatment of myofascial trigger points. Physical Therapy 2000;80(10):997-1003. [PMID: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ (Clinical Research Ed.) 2003;327:555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
Hollen 2005
- Hollen PJ, Gralla RJ, Kris MG, McCoy S, Donaldson GW, Moinpour CM. A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale (LCSS): does format affect patient ratings of symptoms and quality of life? Quality of Life Research 2005;14(3):837-47. [DOI: 10.1007/s11136-004-0833-8] [DOI] [PubMed] [Google Scholar]
Hong 1994
- Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point - the importance of the local twitch response. American Journal of Physical Medicine and Rehabilitation 1994;73(4):256-63. [DOI] [PubMed] [Google Scholar]
Howard 2002
- Smith HS, Audette J, Royal MA. Botulinum toxin in pain management of soft tissue syndromes. Clinical Journal of Pain 2002;18(6):S147-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hubbard 1993
- Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine 1993;18:1803–7. [DOI] [PubMed] [Google Scholar]
Kam 2002
- Kam E, Eslick G, Campbell I. An audit of the effectiveness of acupuncture on musculoskeletal pain in primary health care. Acupuncture in Medicine: Journal of the British Medical Acupuncture Society 2002;20(1):35-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kern 2004
- Kern U, Martin C, Scheicher S, Müller H. Does botulinum toxin A make prosthesis use easier for amputees? Journal of Rehabilitation Medicine 2004;36(5):238-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Langevin 2011
- Langevin P, Peloso PMJ, Lowcock J, Nolan M, Weber J, Gross A, et al. Botulinum toxin for subacute/chronic neck pain. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008626. [DOI: 10.1002/14651858.CD008626] [DOI] [PubMed] [Google Scholar]
Laskin 1969
- Laskin DM. Etiology of the pain-dysfunction syndrome. Journal of the American Dental Association 1969;79:147-53. [DOI] [PubMed] [Google Scholar]
Lew 2002
- Lew MF. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clinical Journal of Pain 2002;18(6):S142-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2003
- Lin TY. Evaluation of a multidisciplinary educational program in patients with disorders musculoskeletal related work (MSDs) [Avaliação de um Programa Educacional Multidisciplinar em Doentes com Dísturbios Ósteo-musculares Relacionados ao trabalho (DORT)]. PhD thesis presented at the Faculty of Medicine of the University of São Paulo 2003.
McMillan 1997
- McMillan AS, Nolan A, Kelly PJ. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. Journal of Orofacial Pain 1997;11:307-14. [PubMed] [Google Scholar]
Peloso 2007
- Peloso PMJ, Gross A, Haines T, Trinh K, Goldsmith CH, Burnie SJ. Medicinal and injection therapies for mechanical neck disorders. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD000319. [DOI: 10.1002/14651858.CD000319.pub4] [DOI] [PubMed] [Google Scholar]
Pereda 2006
- Pereda CA, Jaeger JU, Carmona L. Systematic review: can botulinum toxin be recommended as treatment for pain in myofascial syndrome? [Revisión sistemática: es recomendable el empleo de toxina botulínica como tratamiento del dolor en el síndrome miofascial?]. Systematic Review 2006;2(4):173-82. [DOI] [PubMed] [Google Scholar]
Pettengill 1997
- Pettengill CA, Reisner-Keller L. The use of tricyclic antidepressants for the control of chronic orofacial pain. Cranio 1997;15(1):53-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reilich 2003
- Reilich P, Pongratz D. Myofascial pain syndrome. In: Jost WH, editors(s). Botulinum Toxin in Painful Diseases. Pain Headache. Vol. 14. Basel: Karger, 2003:23-41. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roth 1998
- Roth RS, Horowitz K, Bachman JE. Chronic myofascial pain: knowledge of diagnosis and satisfaction with treatment. Archives of Physical Medicine and Rehabilitation 1998;79(8):966-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Royal 2001
- Royal MA, Gunyea I, Bhakta B, Mowa V, Ward S, Jenson M. Botulinum toxin type A in the treatment of refractory myofascial pain. Neurology 2001;56 Suppl 3:A350. [Google Scholar]
Schiffman 1990
- Schiffman EL, Fricton JR, Haley DP, Shapiro BL. The prevalence and treatment needs of subjects with temporomandibular disorders. Journal of the American Dental Association 1990;120:295-303. [DOI] [PubMed] [Google Scholar]
Sellin 1981
- Sellin LC, Thesleff S. Pre- and post-synaptic actions of botulinum toxin at the rat neuromuscular junction. Journal of Physiology 1981;317:487-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silberstein 2000
- Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 2000;40(6):445–50. [DOI] [PubMed] [Google Scholar]
Simons 1976
- Simons DG. Muscle pain syndromes. Part II. American Journal of Physical Medicine 1976;55(1):15-42. [PubMed] [Google Scholar]
Simons 1996
- Simons DG. Clinical and etiological update of myofascial pain from trigger points. Journal of Musculoskeletal Pain 1996;4(1-2):93-121. [Google Scholar]
Simons 1999
- Simons DG, Travell JG, Simons LS. Travell and Simons' Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd edition. Vol. 1: Upper Half of the Body. Baltimore: Lippincott, Williams & Wilkins, 1999. [Google Scholar]
Simons 2002
- Simons DG, Hong CZ, Simons LS. Endplate potentials are common to mid myofascial trigger points. American Journal of Physical Medicine and Rehabilitation 2002;81:212–22. [DOI] [PubMed] [Google Scholar]
Singer 1997
- Singer E, Dionne R. A controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle pain. Journal of Orofacial Pain 1997;11(2):139-46. [PMID: ] [PubMed] [Google Scholar]
Skootsky 1989
- Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. Western Journal of Medicine 1989;151(2):157-60. [PMC free article] [PubMed] [Google Scholar]
Sola 1955
- Sola AE, Rodenberg ML, Gettys BB. Incidence of hypersensitive areas in posterior shoulder muscles. American Journal of Physical Medicine 1955;34:585-90. [PubMed] [Google Scholar]
Solberg 1986
- Solberg WK. Temporomandibular disorders: masticatory myalgia and its management. British Dental Journal 1986;160:351-6. [DOI] [PubMed] [Google Scholar]
Srbely 2007
- Srbely JZ, Dickey JP. Randomized controlled study of the antinociceptive effect of ultrasound on trigger point sensitivity: novel applications in myofascial therapy? Clinical Rehabilitation 2007;21(5):411-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Talaat 1986
- Talaat AM, el-Dibany MM, el-Garf A. Physical therapy in the management of myofacial pain dysfunction syndrome. Annals of Otology, Rhinology and Laryngology 1986;95(3 Pt 1):225-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tang 2000
- Tang JL, Liu JLY. Misleading funnel plot for detection of bias in meta-analysis. Journal of Clinical Epidemiology 2000;53:477-84. [DOI] [PubMed] [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53:207-16. [DOI] [PubMed] [Google Scholar]
Travell 1999
- Travell D, Simons DG. Myofascial Pain and Dysfunction: the Trigger Point Manual. 2nd edition. Vol. 1: Upper half of the body. Baltimore: Lippincott, Williams & Wilkins, 1999. [Google Scholar]
Tubergen 2002
- Tubergen AV, Debats I, Ryser L, Londoño J, Burgos-Vargas R, Cardiel MH, et al. Use of a numerical rating scale as an answer modality in ankylosing spondylitis–specific questionnaires. Arthritis Care and Research 2002;47(3):242–8. [DOI: 10.1002/art.10397] [DOI] [PubMed] [Google Scholar]
Tunks 1999
- Tunks E, Crook J. Regional soft tissue pains: alias myofascial pain? Baillières Clinical Rheumatology 1999;13:345–70. [PubMed] [Google Scholar]
Unnebrink 2001
- Unnebrink K, Windeler J. Intention-to-treat methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Statistics in Medicine 2001;20:3931-46. [DOI] [PubMed] [Google Scholar]


